Adsorption Performance of Modified Diatomite for Heavy Metal Ions in Wastewater
-
摘要:
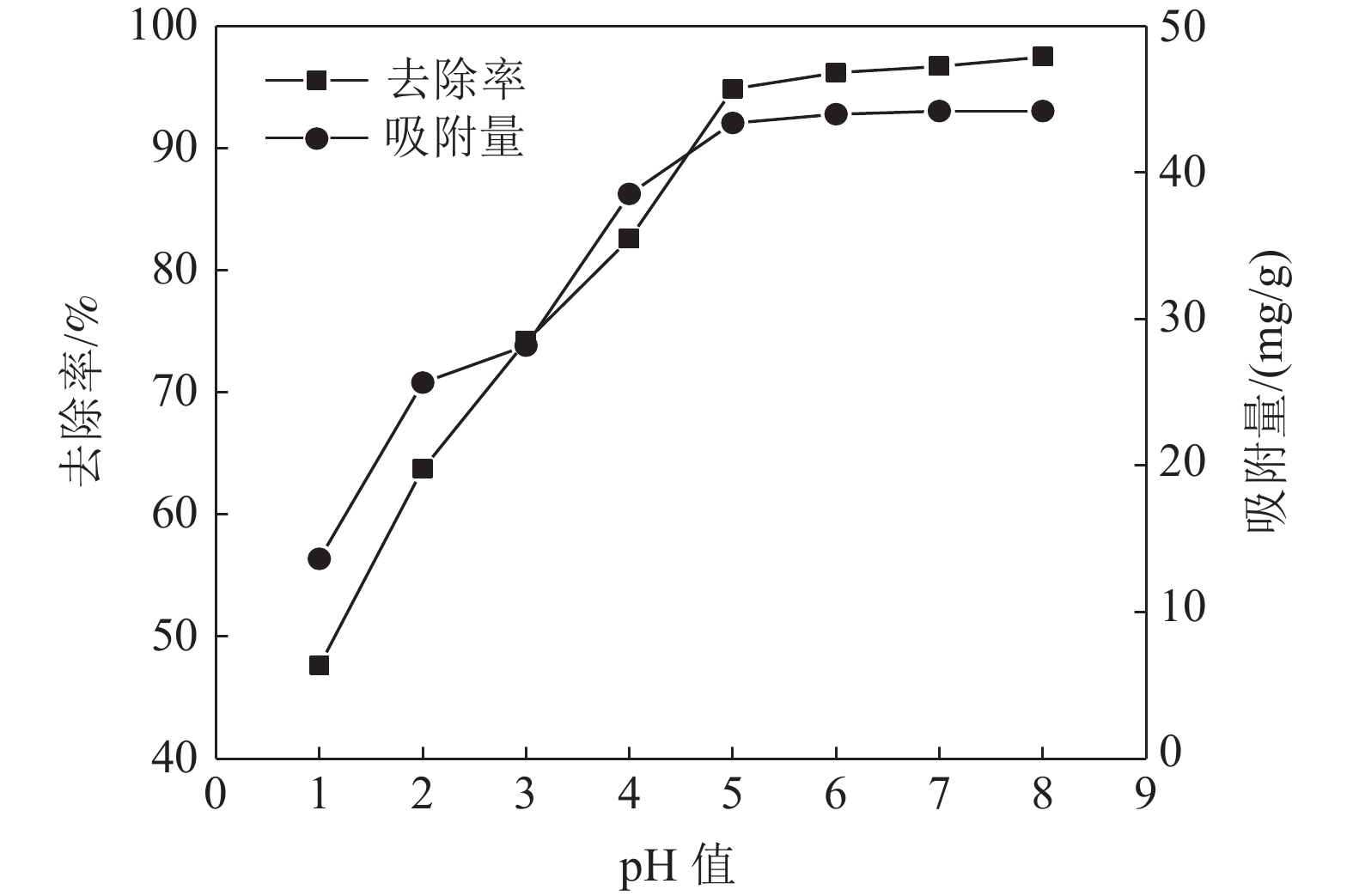
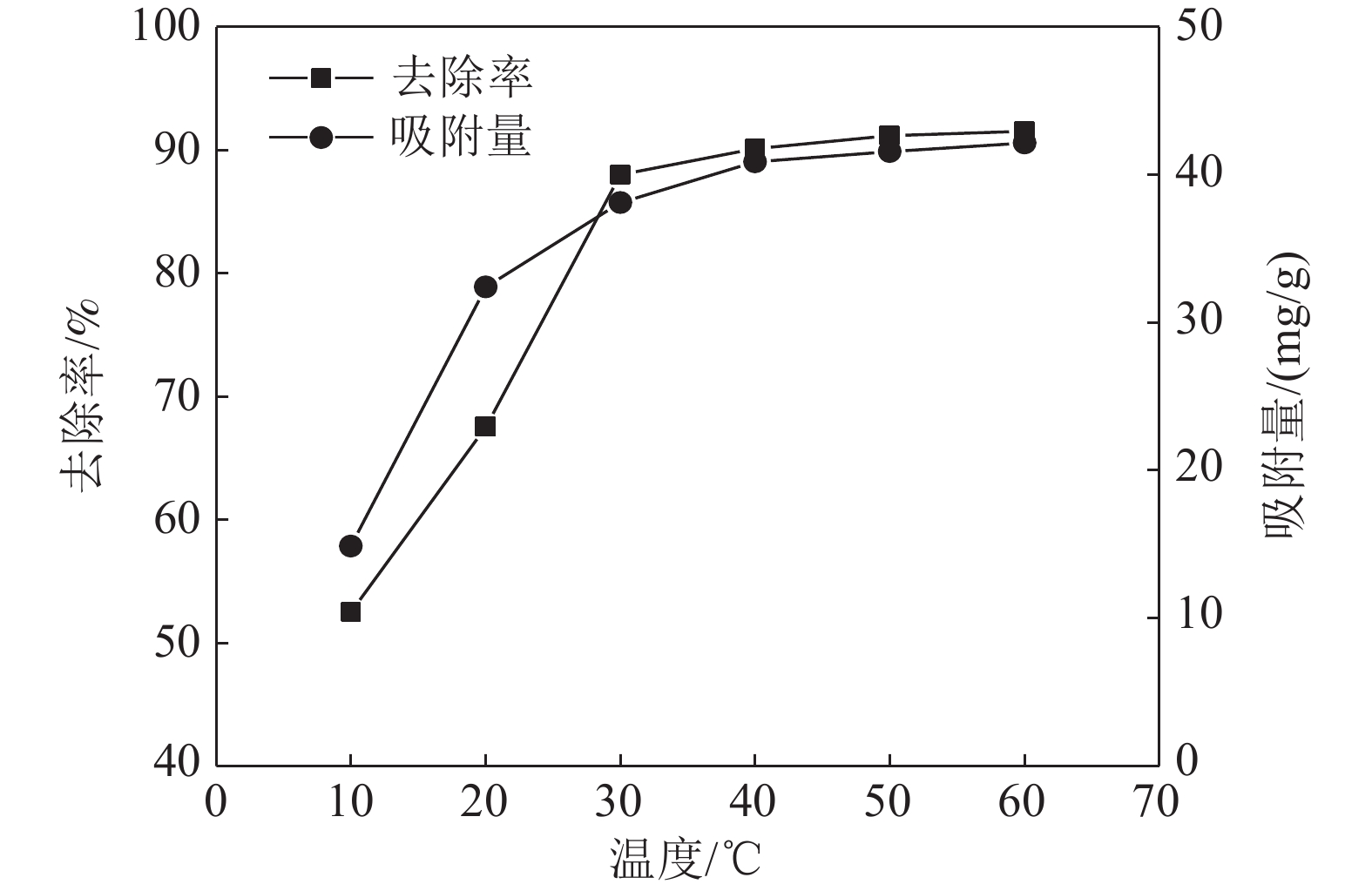
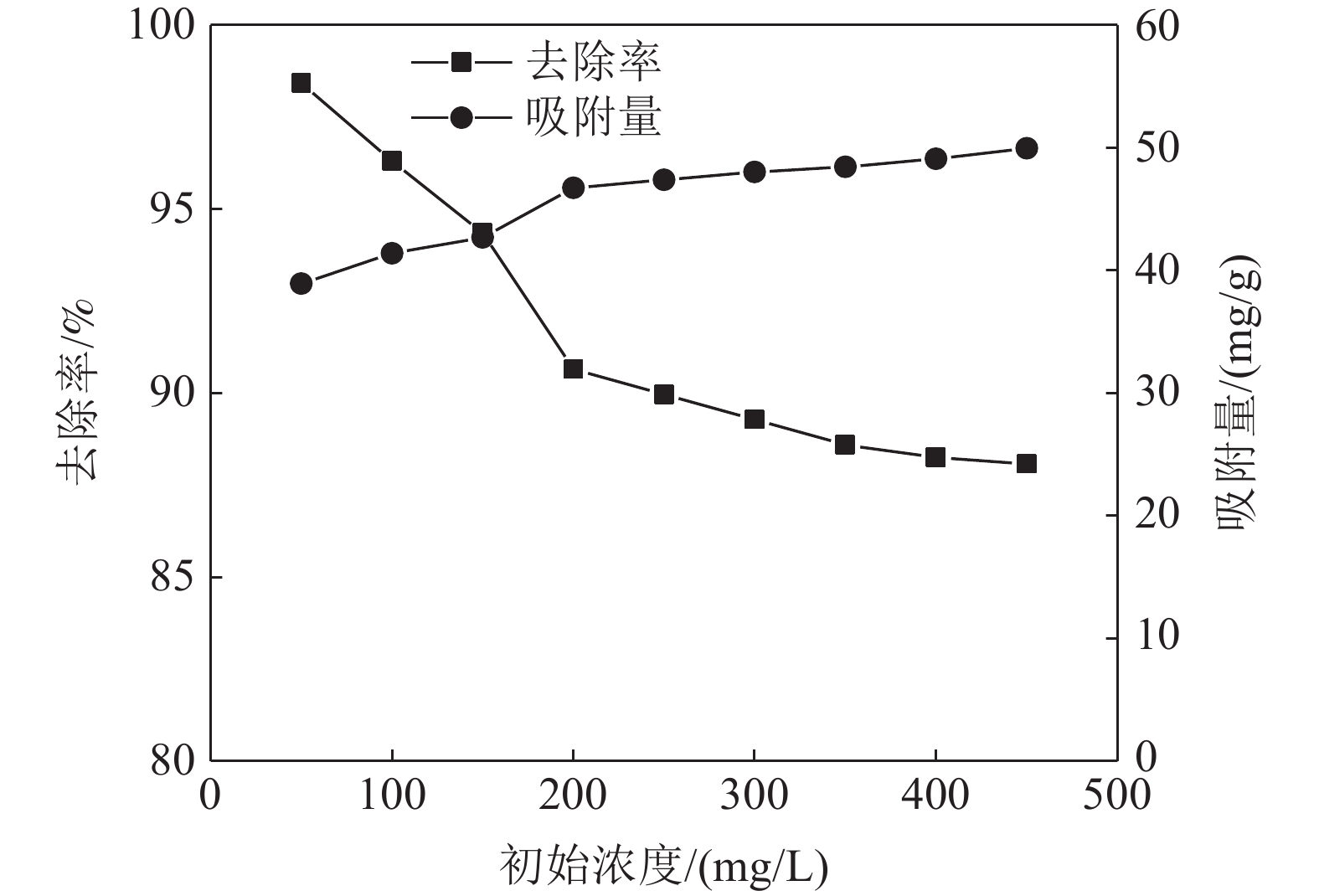
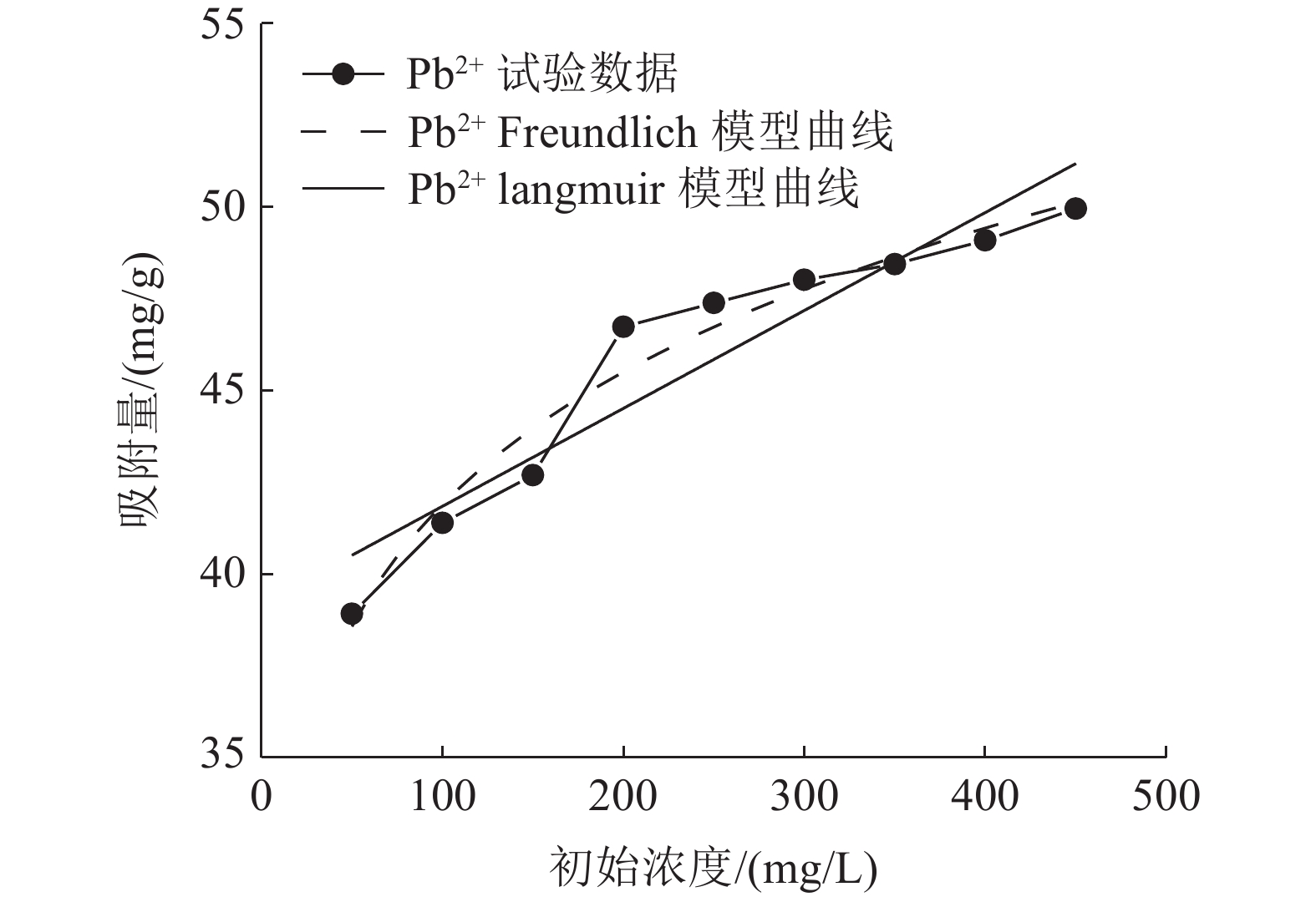
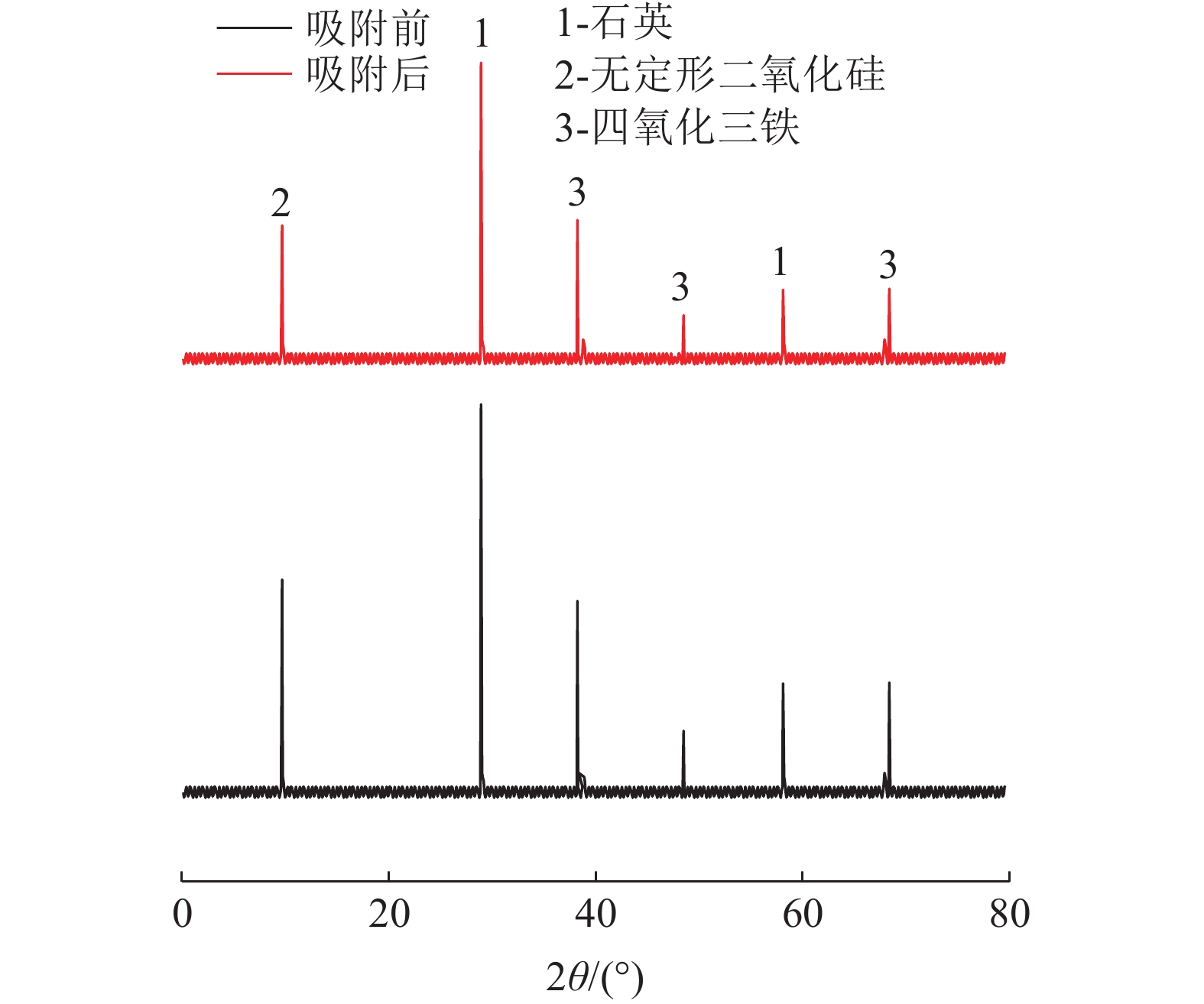
这是一篇矿业工程领域的论文。采用十二烷基磺酸钠和四氧化三铁对硅藻土进行改性,分析外部因素对吸附效果的影响,并开展改性硅藻土吸附重金属离子后物相成分变化以及微观结构变化的实验。结果表明:改性硅藻土掺量为4 g/L、吸附时间为40 min、温度设置为30 ℃,pH值设定为5,初始浓度均设定为200 mg/L时,改性硅藻土的吸附效果达到较佳。Freundlich模型对实验曲线的拟合度为0.90以上,而Langmuir模型对实验曲线的拟合度均在0.9以下,这就说明了Freundlich等温吸附模型更加适用于改性硅藻土吸附重金属铅离子吸附量的变化规律。改性硅藻土吸附铅离子前后的XRD图谱特征衍射峰并未有明显的差异,只是衍射峰的峰强度有所减小,但是改性硅藻土内部其他矿物成分却不变以及结构没有发生明显变化,这也说明了改性硅藻土可以有效地吸附污水中的铅离子,但是吸附过程基本属于物理吸附。
Abstract:This is an article in the field of mining engineering. The diatomite was modified with sodium dodecyl sulfonate and ferroferric oxide, and the influence of external factors on the adsorption effect was analyzed. And the experiment of the phase composition change and the microstructure change after the modified diatomite adsorbs heavy metal ions was carried out. The results show that when the modified diatomite content is 4 g/L, the adsorption time is 40 min, the temperature is set to 30 ℃, the pH value is set to 5, and the initial concentration is set to 200 mg/L, the adsorption of the modified diatomite is relatively good. The fitting degree of the Freundlich model to the test curve is above 0.90, and the fitting degree of the Langmuir model to the test curve is below 0.9, which shows that the Freundlich isotherm adsorption model is more suitable for the change law of the adsorption of heavy metal lead ions on modified diatomite. There is no significant difference in the characteristic diffraction peaks of the XRD pattern before and after the adsorption of lead ions on the modified diatomite. Only the peak intensity of the diffraction peak is reduced. However, the other mineral components inside the modified diatomite remain unchanged and the structure does not change significantly. This also shows that modified diatomaceous earth can effectively adsorb lead ions in sewage. However, the adsorption process is basically physical adsorption.
-

-
[1] 蒋博龙, 史顺杰, 蒋海林, 等. 金属有机框架材料吸附处理苯酚污水机理研究进展[J]. 化工进展, 2021, 40(8):4525-4539.JIANG B L, SHI S J, JIANG H L, et al. Research progress in phenol adsorption mechanism over metal-organic framework from wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(8):4525-4539.
JIANG B L, SHI S J, JIANG H L, et al. Research progress in phenol adsorption mechanism over metal-organic framework from wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(8):4525-4539.
[2] 赵连兵, 先永骏, 文书明, 等. 铅锌选矿废水净化处理研究概述[J]. 矿产综合利用, 2022(3):100-106.ZHAO L B, XIAN Y J, WEN S M, et al. Brief introduction of lead and zinc beneficiation wastewater treatment[J]. Multipurpose Utilization of Mineral Resources, 2022(3):100-106. doi: 10.3969/j.issn.1000-6532.2022.03.018
ZHAO L B, XIAN Y J, WEN S M, et al. Brief introduction of lead and zinc beneficiation wastewater treatment[J]. Multipurpose Utilization of Mineral Resources, 2022(3):100-106. doi: 10.3969/j.issn.1000-6532.2022.03.018
[3] 白玉琦, 安燕, 陈娇, 等. MGO/SA制备及其对含Cr(Ⅵ)污水吸附处理研究[J]. 应用化工, 2021, 50(3):697-701.BAI Y Q, AN Y, CHEN J, et al. Preparation of MGO/SA and study on adsorption of Cr(Ⅵ) in water[J]. Applied Chemical Industry, 2021, 50(3):697-701. doi: 10.3969/j.issn.1671-3206.2021.03.028
BAI Y Q, AN Y, CHEN J, et al. Preparation of MGO/SA and study on adsorption of Cr(Ⅵ) in water[J]. Applied Chemical Industry, 2021, 50(3):697-701. doi: 10.3969/j.issn.1671-3206.2021.03.028
[4] 白润英, 宋博文, 张彧, 等. 一步法La@MgFe2O4的制备及其吸附水中磷的性能[J]. 环境科学, 2021, 42(3):1461-1468.BAI R Y, SONG B W, ZHANG Y, et al. One-step preparation of lanthanum-magnesium ferrite and its phosphate adsorption calpacity in aqueous solutions[J]. Environmental Science, 2021, 42(3):1461-1468.
BAI R Y, SONG B W, ZHANG Y, et al. One-step preparation of lanthanum-magnesium ferrite and its phosphate adsorption calpacity in aqueous solutions[J]. Environmental Science, 2021, 42(3):1461-1468.
[5] 王昊, 赵霞, 李博文, 等. 微生物吸附固定化技术在污水处理中的研究进展[J]. 应用化工, 2020, 49(9):2341-2345.WANG H, ZHAO X, LI B W, et al. Advances in the study of adsorptive immobilized microorganisms in wastewater treatment[J]. Applied Chemical Industry, 2020, 49(9):2341-2345. doi: 10.3969/j.issn.1671-3206.2020.09.044
WANG H, ZHAO X, LI B W, et al. Advances in the study of adsorptive immobilized microorganisms in wastewater treatment[J]. Applied Chemical Industry, 2020, 49(9):2341-2345. doi: 10.3969/j.issn.1671-3206.2020.09.044
[6] 马立群, 李爽, 王雅珍, 等. 污水中重金属离子吸附材料研究进展[J]. 化工新型材料, 2020, 48(10):290-293.MA L Q, LI S, WANG Y Z, et al. Research progress on adsorption material of heavy metal ions in sewage[J]. New Chemical Materials, 2020, 48(10):290-293.
MA L Q, LI S, WANG Y Z, et al. Research progress on adsorption material of heavy metal ions in sewage[J]. New Chemical Materials, 2020, 48(10):290-293.
[7] 闫英师, 李玉凤, 赵礼兵. 改性钢渣吸附重金属离子的研究现状[J]. 矿产综合利用, 2021(1):8-13.YAN Y S, LI Y F, ZHAO L B. Research status of heavy metal ions adsorption by modified steel slag[J]. Multipurpose Utilization of Mineral Resources, 2021(1):8-13 doi: 10.3969/j.issn.1000-6532.2021.01.002
YAN Y S, LI Y F, ZHAO L B. Research status of heavy metal ions adsorption by modified steel slag[J]. Multipurpose Utilization of Mineral Resources, 2021(1):8-13 doi: 10.3969/j.issn.1000-6532.2021.01.002
[8] 杜璨, 左锐, 马啸, 等. 典型吸附材料对含Cs废水的吸附效能对比研究[J]. 北京师范大学学报(自然科学版), 2020, 56(2):188-194.DU C, ZUO R, MA X, et al. Adsorption of cesium-containing wastewater by typical materials[J]. Journal of Beijing Normal University(Natural Science), 2020, 56(2):188-194.
DU C, ZUO R, MA X, et al. Adsorption of cesium-containing wastewater by typical materials[J]. Journal of Beijing Normal University(Natural Science), 2020, 56(2):188-194.
[9] 胡潇, 梁虎南, 于大禹. 硅藻土处理染料废水的研究进展[J]. 硅酸盐通报, 2018, 37(1):160-165.HU X, LIANG H N, YU D Y. Research progress of diatomite used in dye wastewater treatment[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(1):160-165.
HU X, LIANG H N, YU D Y. Research progress of diatomite used in dye wastewater treatment[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(1):160-165.
[10] 董超超, 邓小川, 王斌, 等. 以硅藻土为原料制备铯吸附剂及其吸附性能初探[J]. 无机盐工业, 2021, 53(6):134-139.DONG C C, DENG X C, WANG B, et al. Preparation of cesium adsorbent from diatomite and its adsorption properties[J]. Inorganic Chemicals Industry, 2021, 53(6):134-139.
DONG C C, DENG X C, WANG B, et al. Preparation of cesium adsorbent from diatomite and its adsorption properties[J]. Inorganic Chemicals Industry, 2021, 53(6):134-139.
[11] 林志茂, 鲜东帆, 周万强, 等. U(Ⅵ)在缓冲回填材料高庙子膨润土胶体上的吸附[J]. 核化学与放射化学, 2021, 43(2):176-182.LIN Z M, XIAN D F, ZHOU W Q, et al. Investigation of U (Ⅵ) sorption on GMZ bentonite colloid[J]. Journal of Nuclear and Radiochemistry, 2021, 43(2):176-182. doi: 10.7538/hhx.2020.YX.2019039
LIN Z M, XIAN D F, ZHOU W Q, et al. Investigation of U (Ⅵ) sorption on GMZ bentonite colloid[J]. Journal of Nuclear and Radiochemistry, 2021, 43(2):176-182. doi: 10.7538/hhx.2020.YX.2019039
[12] 胡啸龙, 孟昭福, 王新欣, 等. 双子型复配增强两性膨润土吸附Cr(VI)的效应[J]. 中国环境科学, 2020, 40(5):2087-2094.HU X L, MENG Z F, WANG X X, et al. Enhancement of the effect of amphoteric bentonite on Cr(VI) adsorption by baryonic complexes[J]. China Environmental Science, 2020, 40(5):2087-2094. doi: 10.3969/j.issn.1000-6923.2020.05.027
HU X L, MENG Z F, WANG X X, et al. Enhancement of the effect of amphoteric bentonite on Cr(VI) adsorption by baryonic complexes[J]. China Environmental Science, 2020, 40(5):2087-2094. doi: 10.3969/j.issn.1000-6923.2020.05.027
-



 下载:
下载: