N235 Recovers Vanadium from Waste Solution of Vanadium Precipitation by Spent Catalyst
-
摘要:
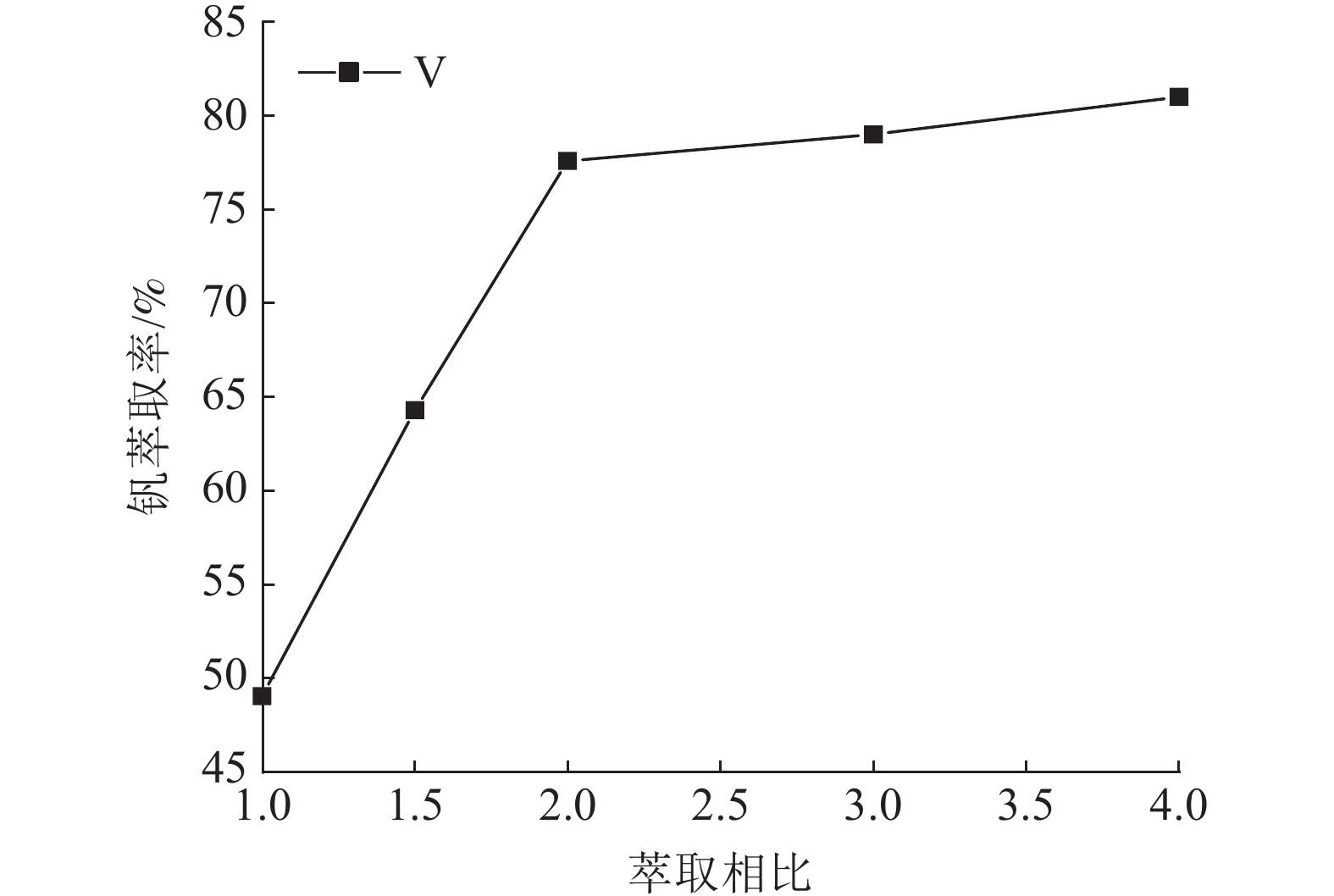
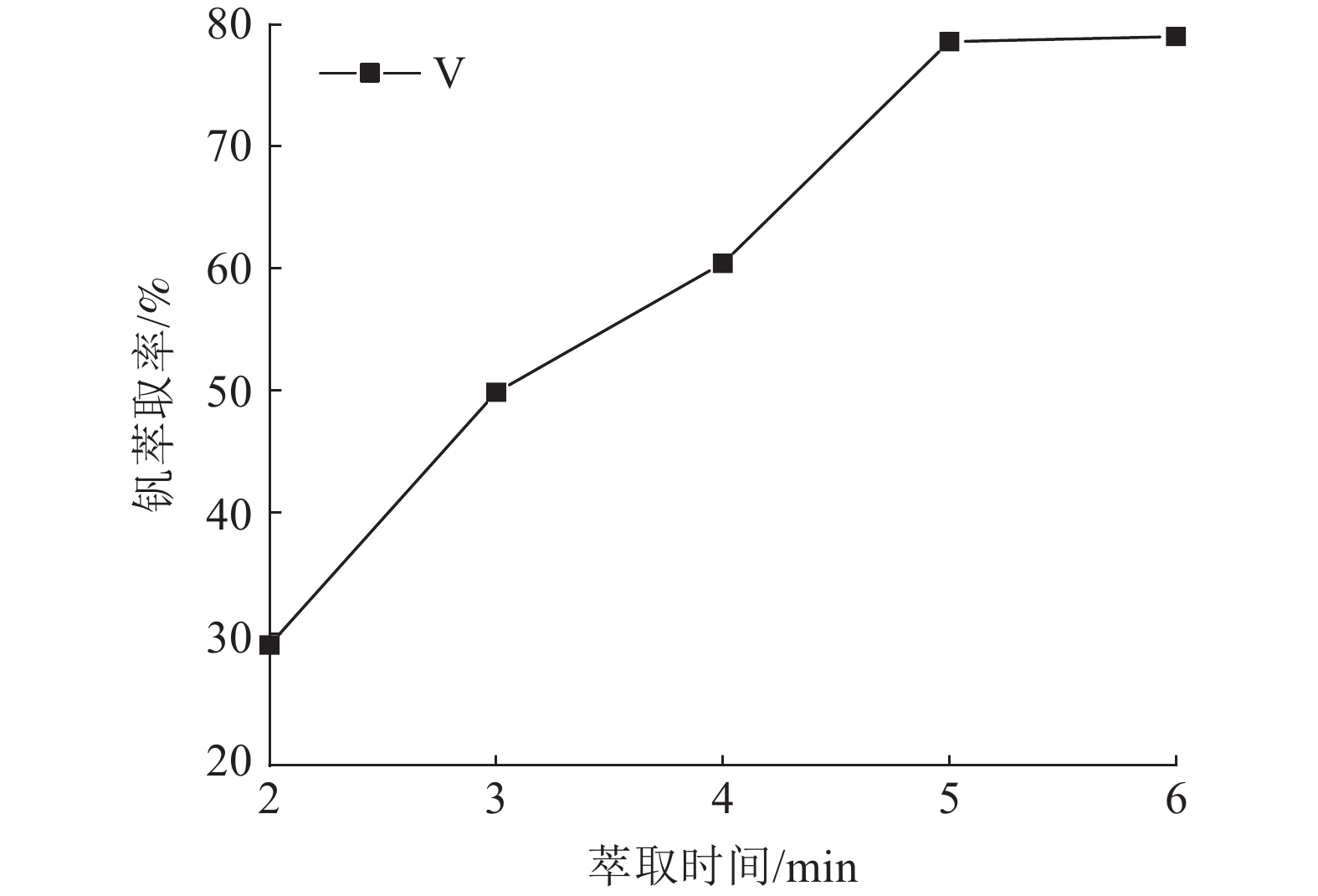
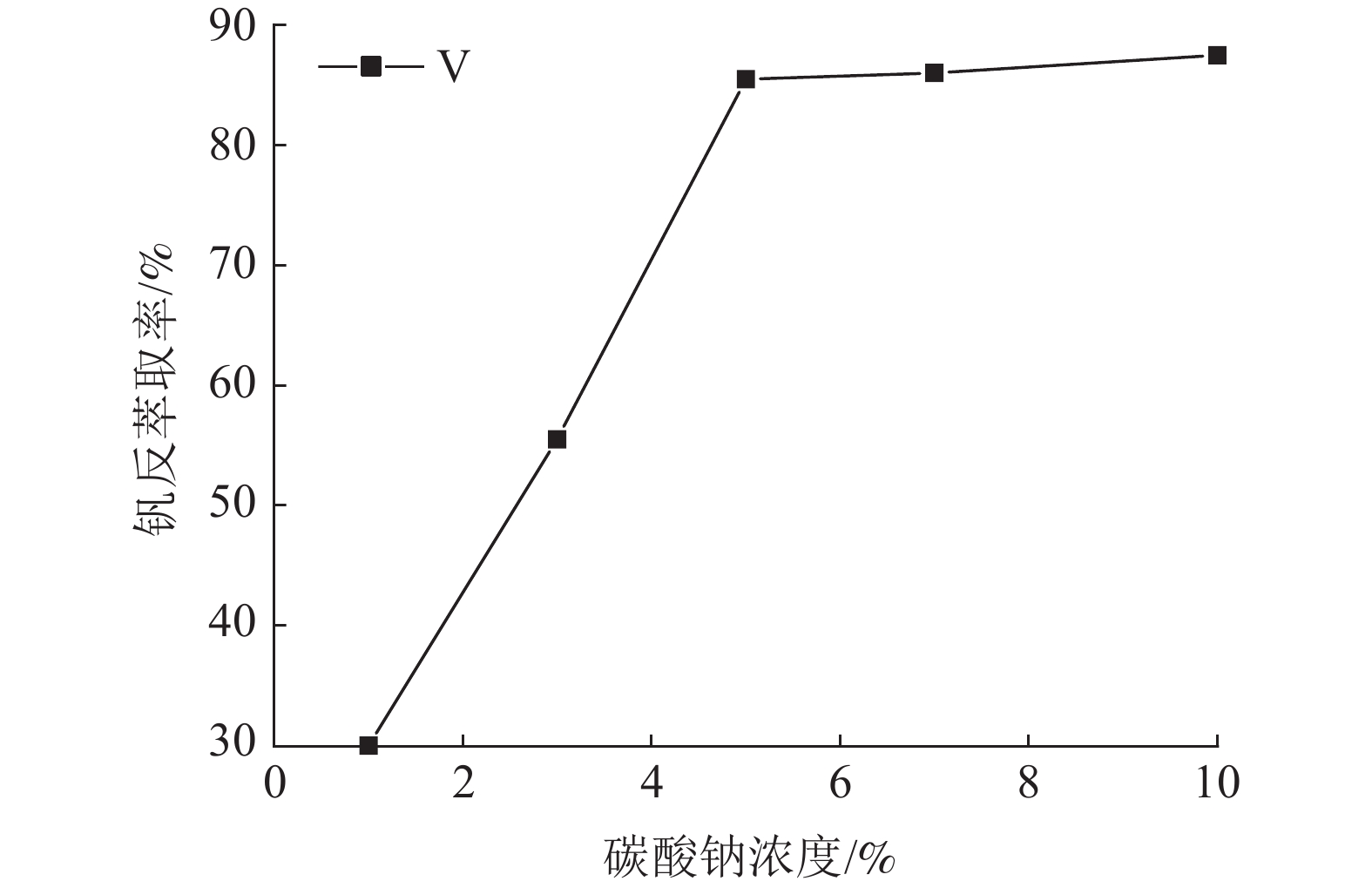
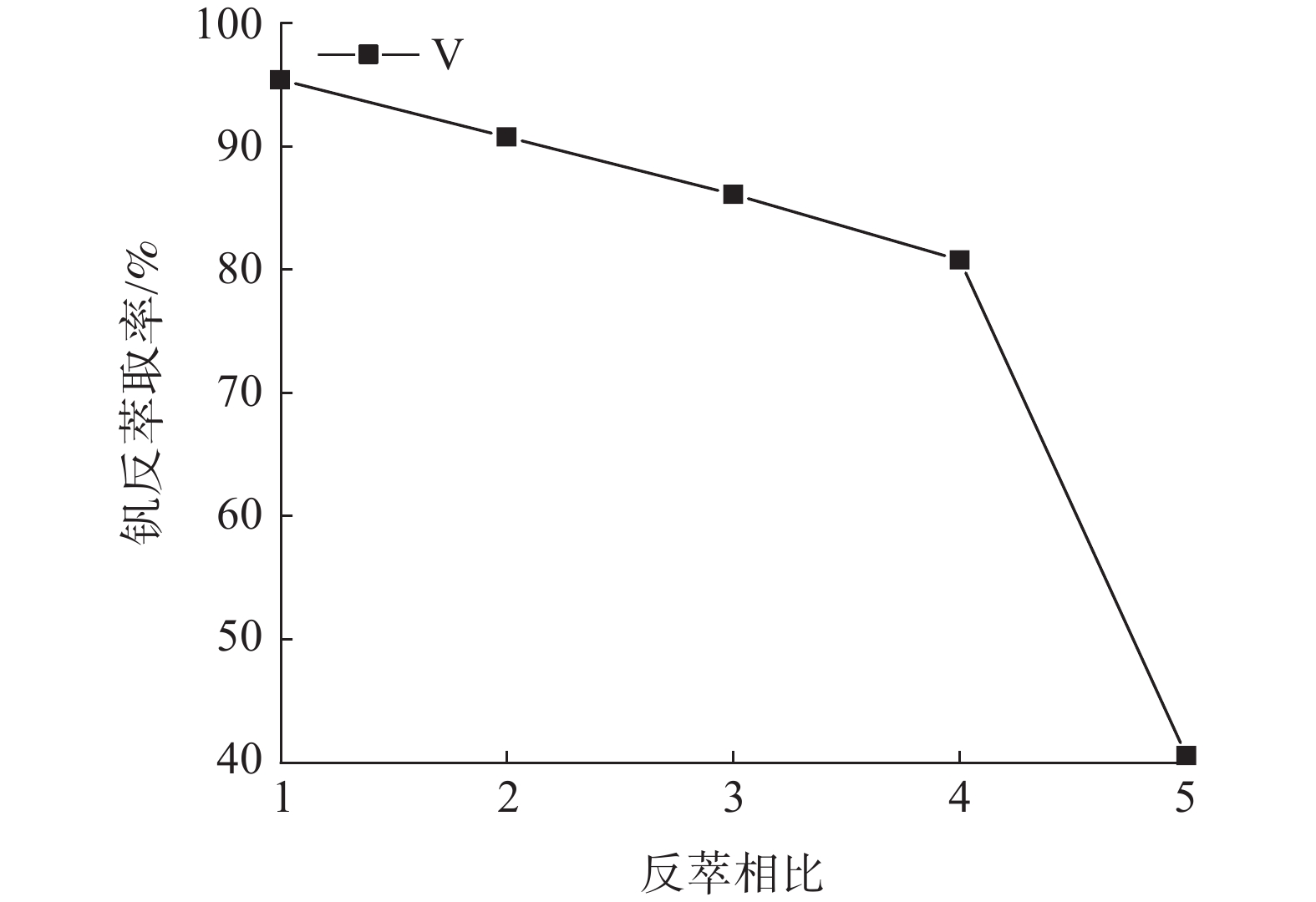
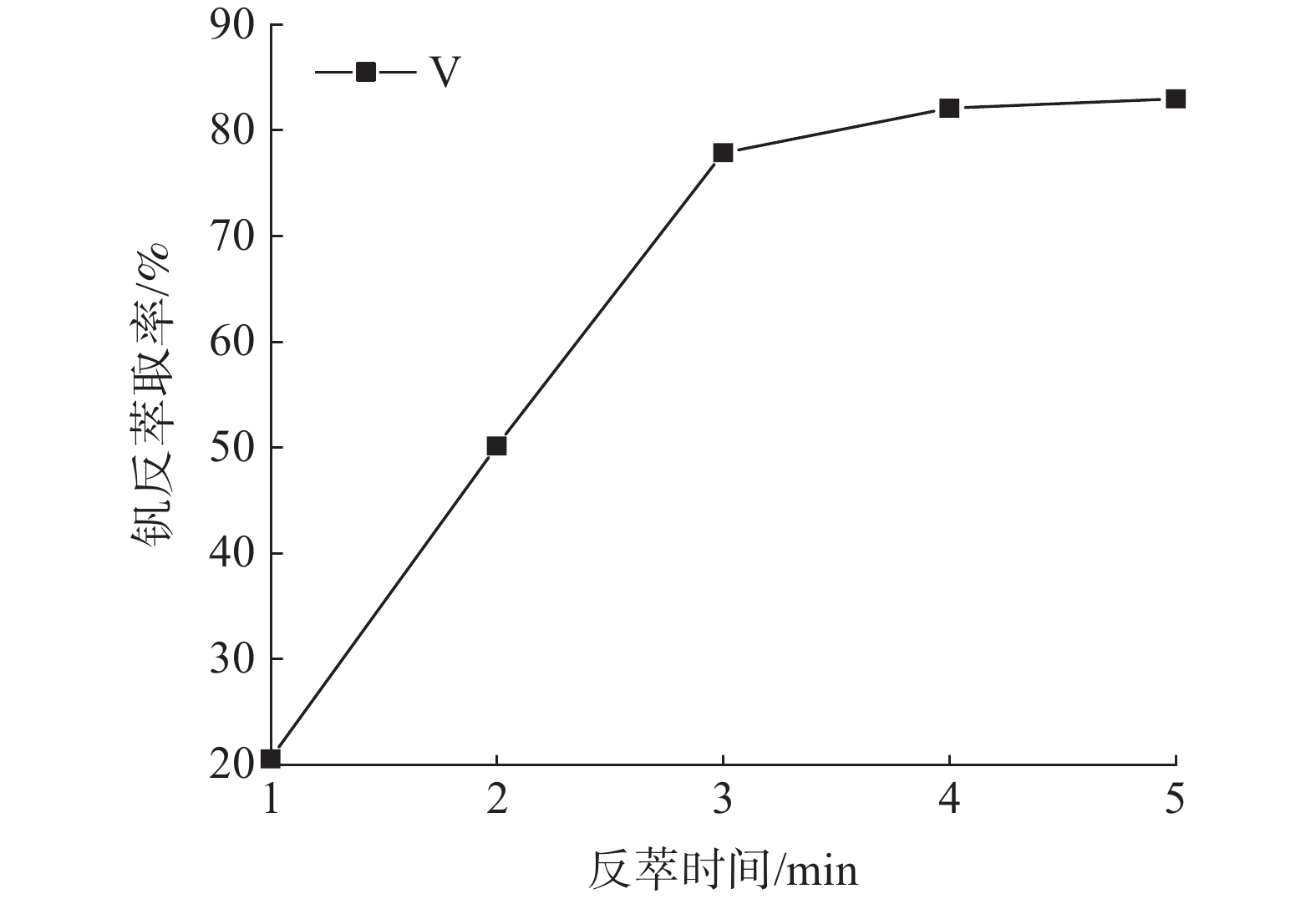
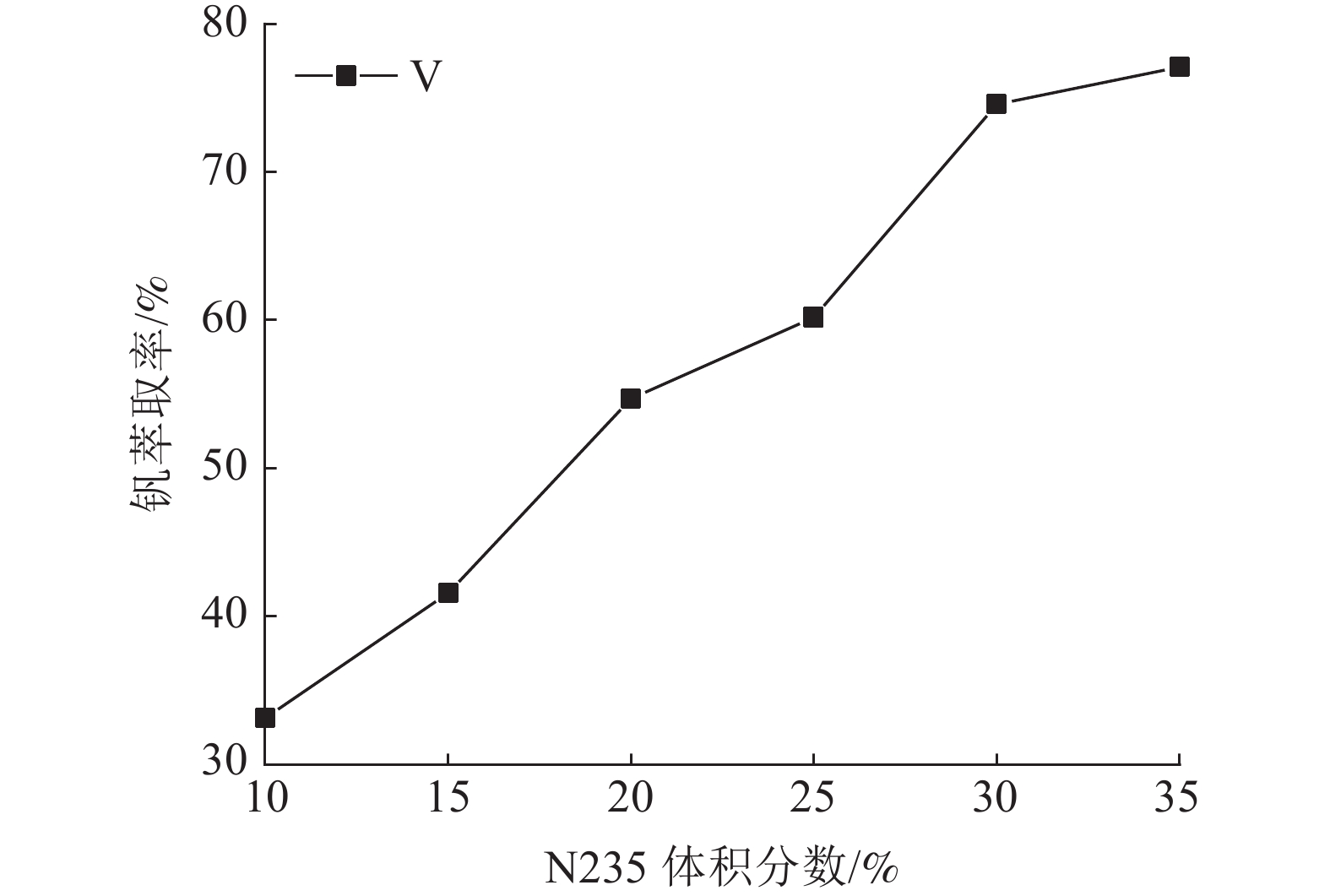
这是一篇冶金工程领域的论文。针对陕西某炼锌厂废催化剂的沉钒废液,通过溶剂萃取法除杂并回收沉钒废液中的钒,考查N235体积分数、萃取相比、萃取时间、萃取级数等对钒萃取率的影响。结果表明:采用30%N235+5%TBP+65%的磺化煤油的萃取体系经过四级逆流萃取,萃取相比VO/VA=2∶1,萃取时间t=5 min,钒的萃取率为95.68%;反萃取剂Na2CO3体积分数为5%,反萃相比V'O/V'A=4∶1,反萃时间t=4 min,经三级逆流反萃取,钒的反萃取率为98.25%。
Abstract:This is an article in the field of metallurgical engineering. For the vanadium precipitation waste liquid of a waste catalyst in a zinc smelter in Shaanxi, the vanadium in the vanadium precipitation waste liquid was recovered by solvent extraction method. The effects of N235 volume fraction, extraction ratio, extraction time and extraction stage on the vanadium extraction rate were investigated. The results showed that the extraction system with 30%N235+5%TBP+65% sulfonated kerosene was used for four-stage countercurrent extraction, the extraction ratio of VO/VA= 2∶1, the extraction time was t=5 min, and the extraction rate of vanadium was 95.68%. The volume fraction of Na2CO3 was 5%, the extraction ratio of V'o/V'A =4∶1, the extraction time was t=4 min, and the extraction rate of vanadium was 98.25% after three-stage counter-current extraction.
-
Key words:
- Metallurgical engineering /
- Heavy vanadium waste liquid /
- Vanadium /
- Extraction /
- N235 /
- Sulfonated kerosene
-

-
表 1 主要化学成分/(g/L)
Table 1. Main chemical constituents
V TFe K+ Na+ Zn2+ 10.23 30.41 2.59 1.83 12.79 -
[1] 洪颖, 郭双华, 李雨, 等. 提钒技术研究进展[J]. 广州化工, 2021, 49(17):23-25.HONG Y, GUO S H, LI Y, et al. Research progress on extraction technology for vanadium[J]. Guangzhou Chemical Industry, 2021, 49(17):23-25. doi: 10.3969/j.issn.1001-9677.2021.17.008
HONG Y, GUO S H, LI Y, et al. Research progress on extraction technology for vanadium[J]. Guangzhou Chemical Industry, 2021, 49(17):23-25. doi: 10.3969/j.issn.1001-9677.2021.17.008
[2] 李昌林. 难处理石煤提钒工艺及相关理论研究[D]. 长沙: 中南大学, 2011.LI C L. Research on vanadium extraction technology from refractory stone coal and related theories[D]. Changsha: Central South University, 2011.
LI C L. Research on vanadium extraction technology from refractory stone coal and related theories[D]. Changsha: Central South University, 2011.
[3] 包申旭, 张一敏, 刘涛, 等. 全球钒的生产、消费及市场分析[J]. 中国矿业, 2009, 18(7):12-15.BAO S X, ZHANG Y M, LIU T, et al. The production, consumption and market analysis of vanadium in the world[J]. China Mining Magazine, 2009, 18(7):12-15. doi: 10.3969/j.issn.1004-4051.2009.07.004
BAO S X, ZHANG Y M, LIU T, et al. The production, consumption and market analysis of vanadium in the world[J]. China Mining Magazine, 2009, 18(7):12-15. doi: 10.3969/j.issn.1004-4051.2009.07.004
[4] 赵海燕. 钒资源利用概况及我国钒市场需求分析[J]. 矿产保护与利用, 2014(2):54-58.ZHAO H Y. Analysis of vanadium resources utilization and demand for vanadium in China[J]. Conservation and Utilization of Mineral Resources, 2014(2):54-58
ZHAO H Y. Analysis of vanadium resources utilization and demand for vanadium in China[J]. Conservation and Utilization of Mineral Resources, 2014(2):54-58
[5] 徐正震, 梁精龙, 李慧, 等. 含钒废弃物中钒的回收研究现状及展望[J]. 矿产综合利用, 2020(3):8-13.XU Z Z, LIANG J L, LI H, et al. Research status and prospects of vanadium recovery in vanadium containing wastes[J]. Multipurpose Utilization of Mineral Resources, 2020(3):8-13. doi: 10.3969/j.issn.1000-6532.2020.03.002
XU Z Z, LIANG J L, LI H, et al. Research status and prospects of vanadium recovery in vanadium containing wastes[J]. Multipurpose Utilization of Mineral Resources, 2020(3):8-13. doi: 10.3969/j.issn.1000-6532.2020.03.002
[6] 赵备备, 李兰杰, 柳林, 等. 废钒触媒提钒工艺研究[J]. 矿产综合利用, 2019(6):80-83.ZHAO B B, LI L J, LIU L, et al. Study on vanadium extraction from waste vanadium catalyst[J]. Multipurpose Utilization of Mineral Resources, 2019(6):80-83. doi: 10.3969/j.issn.1000-6532.2019.06.018
ZHAO B B, LI L J, LIU L, et al. Study on vanadium extraction from waste vanadium catalyst[J]. Multipurpose Utilization of Mineral Resources, 2019(6):80-83. doi: 10.3969/j.issn.1000-6532.2019.06.018
[7] 程倩, 王明, 宁新霞, 等. 从某低品位炭质钒矿石中酸浸-萃取-氨沉淀提钒实验研究[J]. 矿产综合利用, 2021(3):17-21.CHENG Q, WANG M, NING X X, et al. Experimental study on extraction of vanadium by acid leaching - extraction - ammonia precipitation from a low grade carbonaceous vanadium ore[J]. Multipurpose Utilization of Mineral Resources, 2021(3):17-21.
CHENG Q, WANG M, NING X X, et al. Experimental study on extraction of vanadium by acid leaching - extraction - ammonia precipitation from a low grade carbonaceous vanadium ore[J]. Multipurpose Utilization of Mineral Resources, 2021(3):17-21.
[8] Zeng L, Cheng C Y. A literature review of the recovery of molybdenum and vanadium from spent[J]. Hydrometallurgy, 2009, 98(1-2):10-20. doi: 10.1016/j.hydromet.2009.03.012
[9] Shi Q H, Zhang Y M, Huang J, et al. Synergistic solvent extraction of vanadium from leaching solution of stone coal using D2EHPA and PC88A[J]. Separation and Purification Technology, 2017, 181:1-7. doi: 10.1016/j.seppur.2017.03.010
[10] 胡艺博, 叶国华, 左琪, 等. 石煤钒矿酸浸液中萃取提钒的研究进展与前景[J]. 矿产综合利用, 2020(1):10-15.HU Y B, YE G H, ZUO Q, et al. Research progress and prospect of vanadium extraction from acid leaching solution of stone coal vanadium ore[J]. Multipurpose Utilization of Mineral Resources, 2020(1):10-15. doi: 10.3969/j.issn.1000-6532.2020.01.002
HU Y B, YE G H, ZUO Q, et al. Research progress and prospect of vanadium extraction from acid leaching solution of stone coal vanadium ore[J]. Multipurpose Utilization of Mineral Resources, 2020(1):10-15. doi: 10.3969/j.issn.1000-6532.2020.01.002
[11] Feng Y L, Wang J F, Xu X F. Extraction of palladium by N7301[J]. Non-ferrous Metal, 1998, 50(1):76-79.
-



 下载:
下载: