Integrated Leaching of Cobalt Hydroxide Intermediate and Nickel-cobalt Sulfide Intermediate
-
摘要:
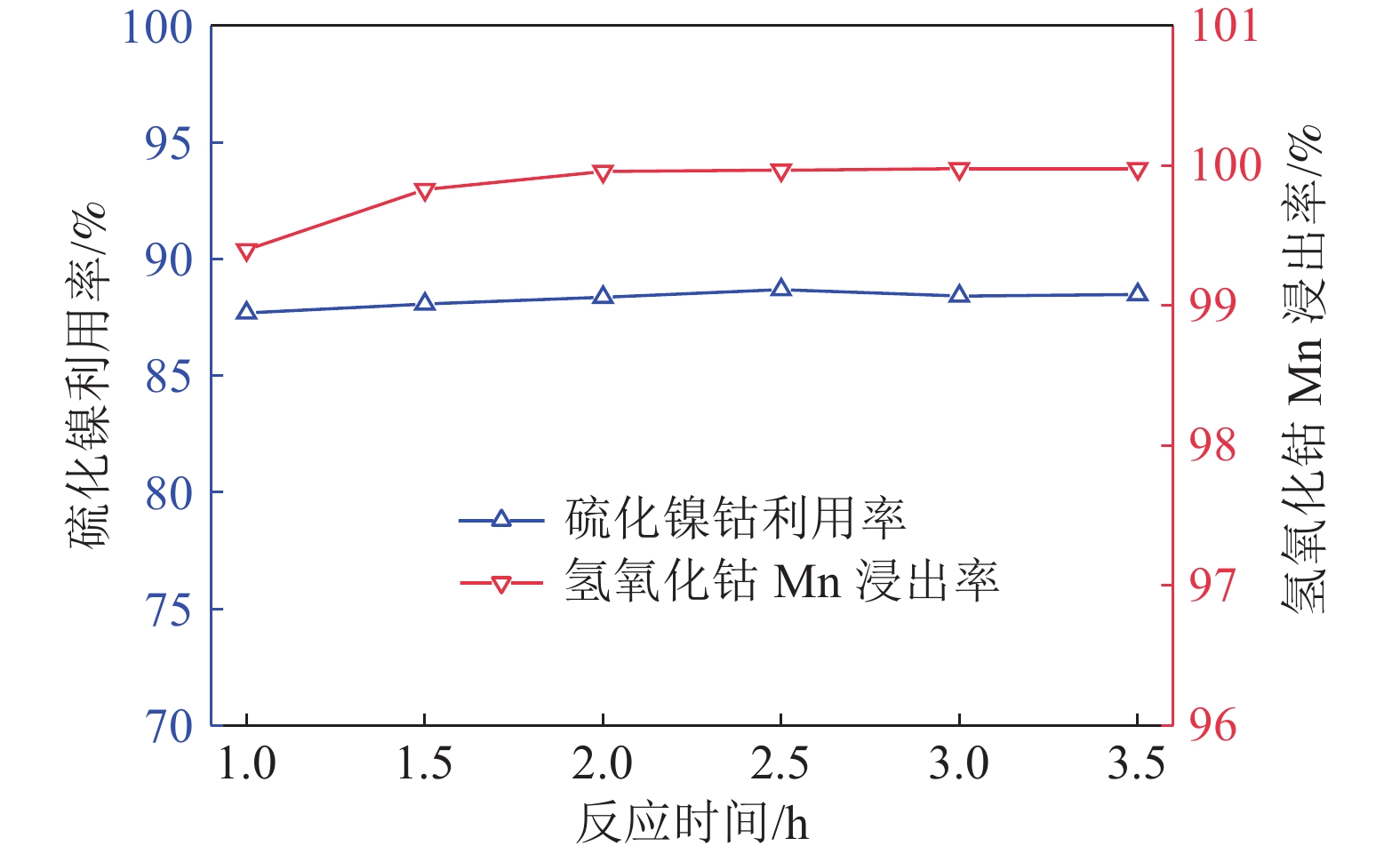
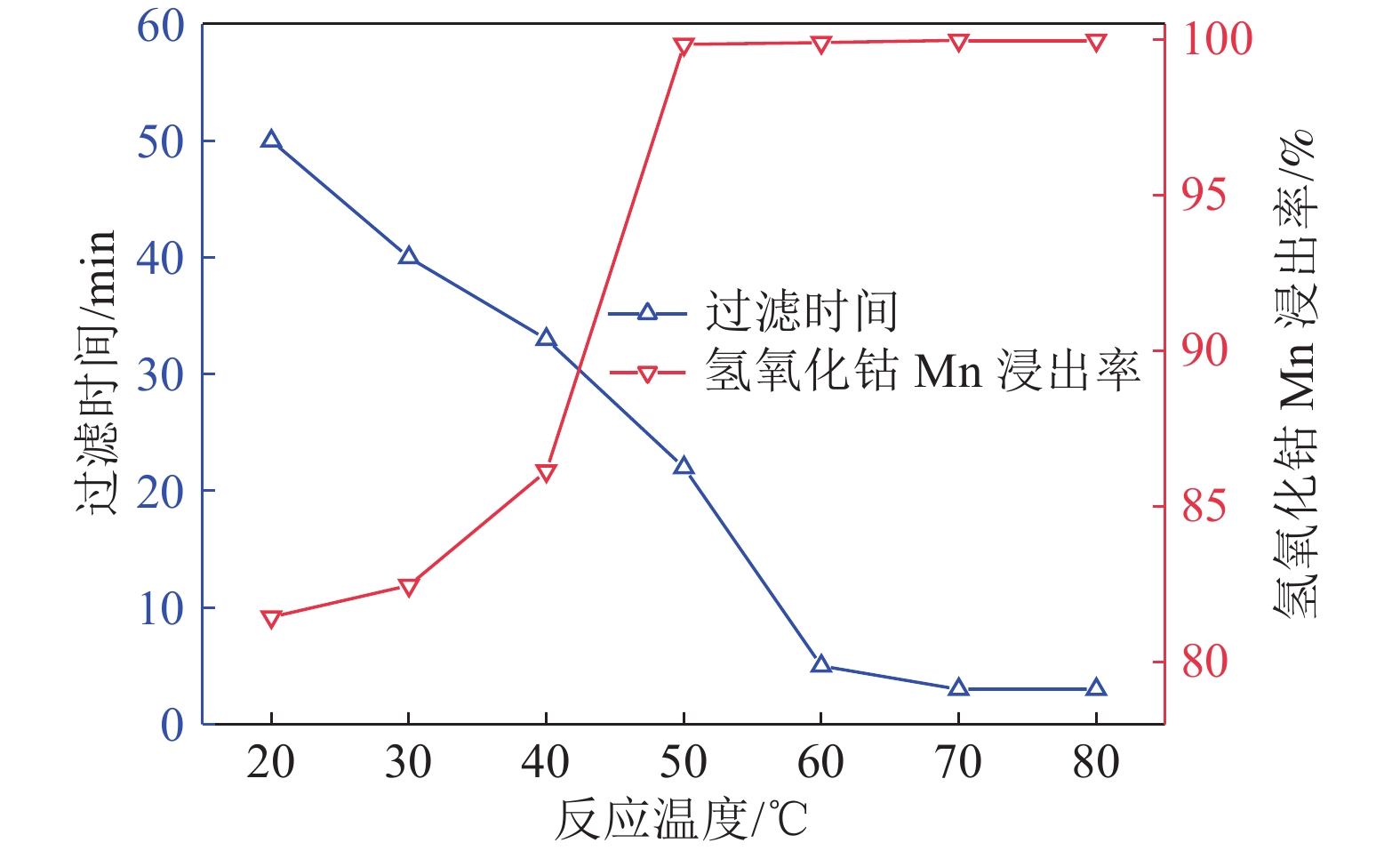
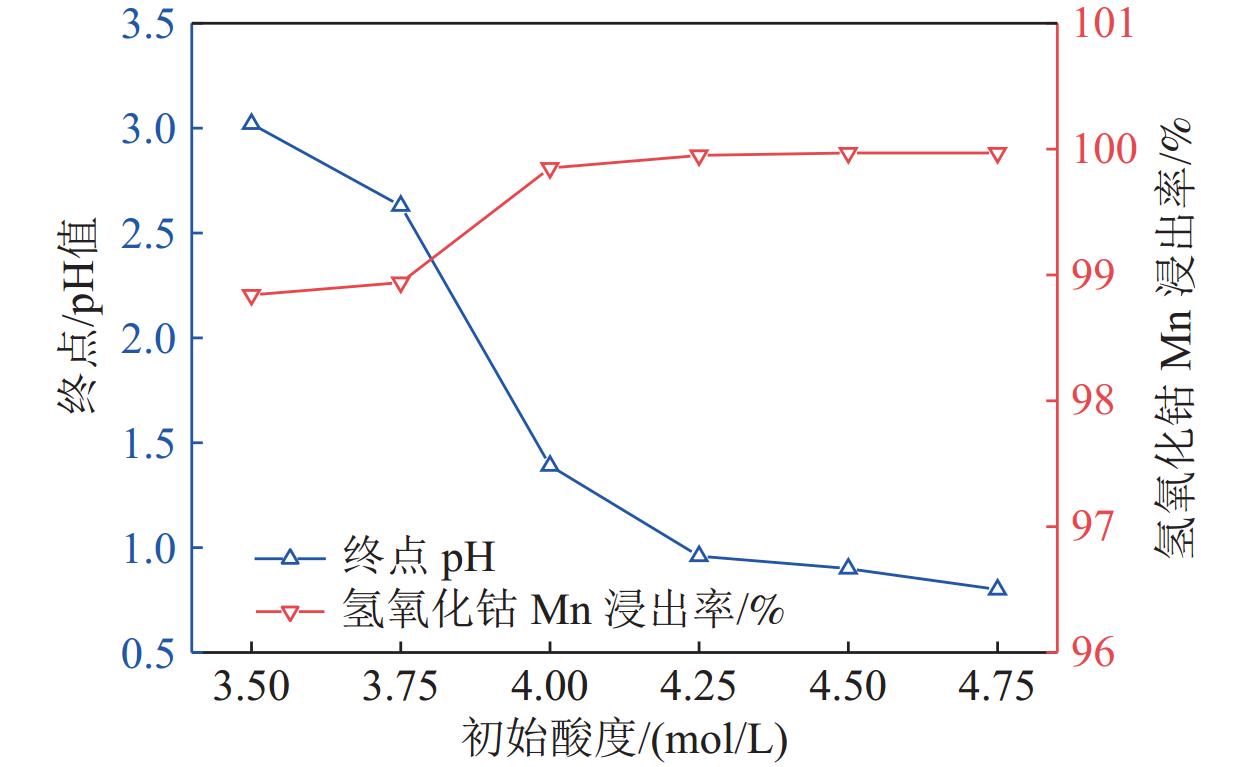
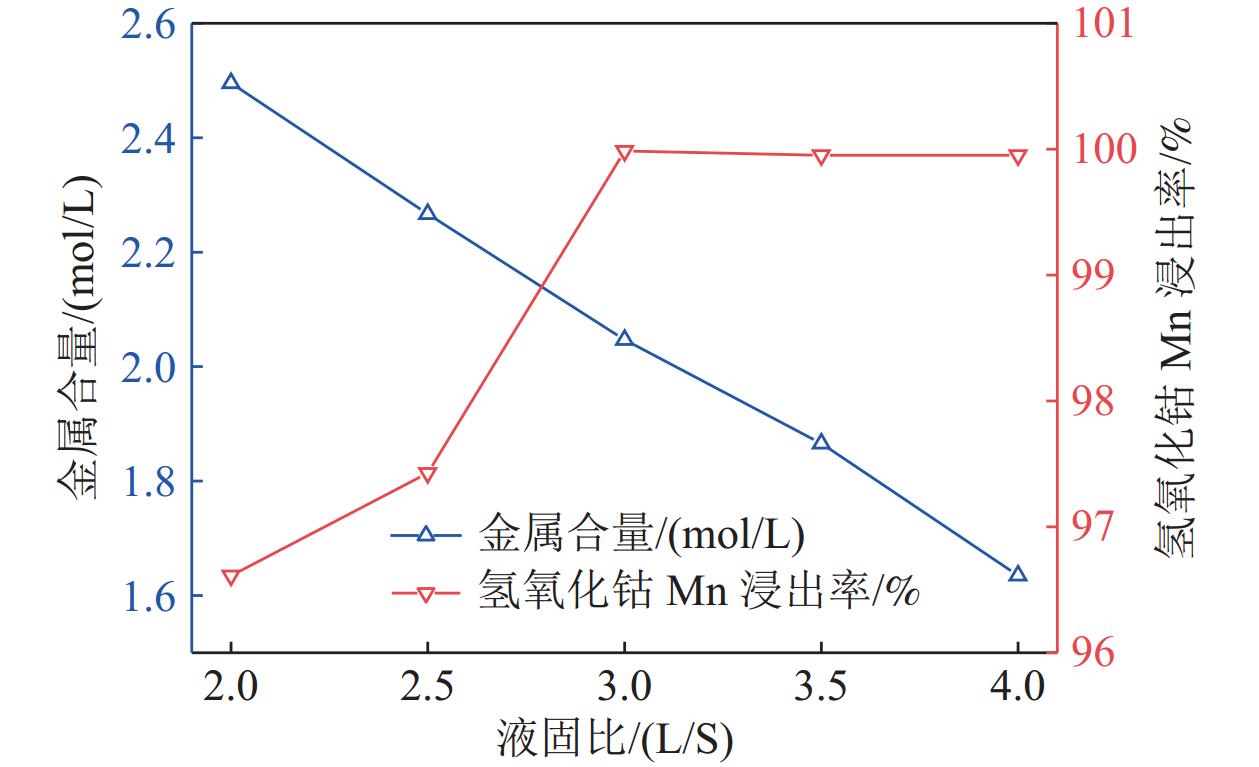
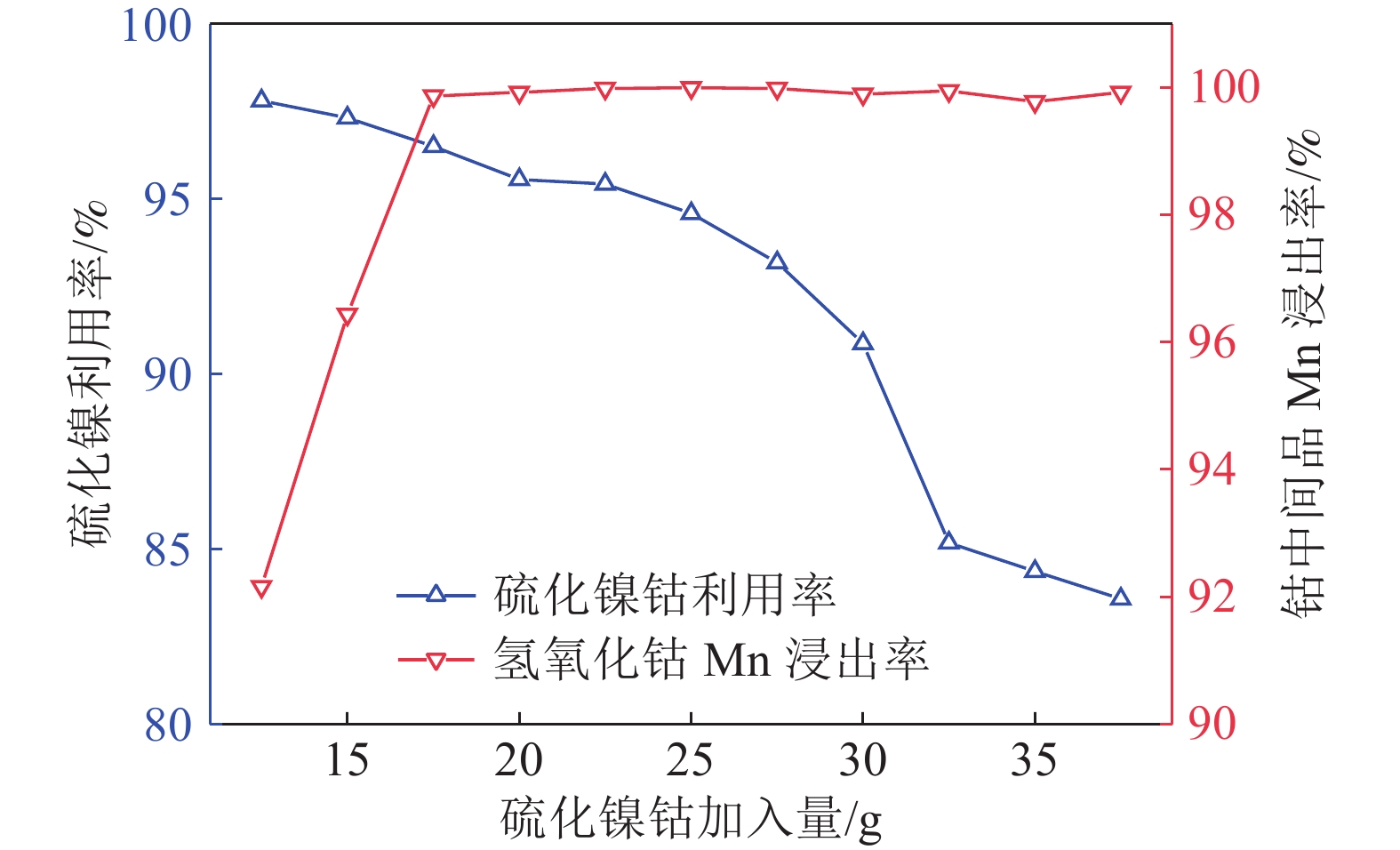
研究了氢氧化钴中间品和硫化镍钴中间品联合氧化还原浸出,在较佳反应条件下,硫化镍钴中间品与氢氧化钴中间品质量比1/5、反应初始酸度为4 mol/L、反应温度为70 ℃、反应液固比为3∶1、反应时间为2.5 h,氢氧化钴中间品中锰的浸出率和硫化镍钴中间品的利用率分别达到99.92%和90.65%。本工艺避免了氢氧化钴中间品单独浸出的还原剂消耗和硫化镍钴中间品单独浸出的氧化剂消耗,实现了氢氧化钴中间品和硫化镍钴中间品协同浸出,且操作简便,适于工业生产应用。
Abstract:The integrated leaching of cobalt hydroxide intermediate and nickel-cobalt sulfide intermediate was studied. The experimental results show that the recovery of manganese in cobalt hydroxide intermediate and the utilization of nickel and cobalt sulfide intermediate were 99.92% and 90.65%, respectively, at the optimum conditions including quality ratio of cobalt hydroxide intermediate and nickel-cobalt sulfide intermediate of 1/5, initial acidity of reaction of 4 mol/L, reaction temperature of 70 ℃, liquid-solid reaction ratio of 3:1 and reaction time of 2.5 h. The residue could be used for leaching cobalt hydroxide intermediate as reductant. The objective of integrated leaching of cobalt hydroxide intermediate and nickel-cobalt sulfide intermediate was attained without the addition of any other oxygenant or reductant. Most importantly, the integrated process leaching is simple and feasible to operate and suitable for industrial production.
-

-
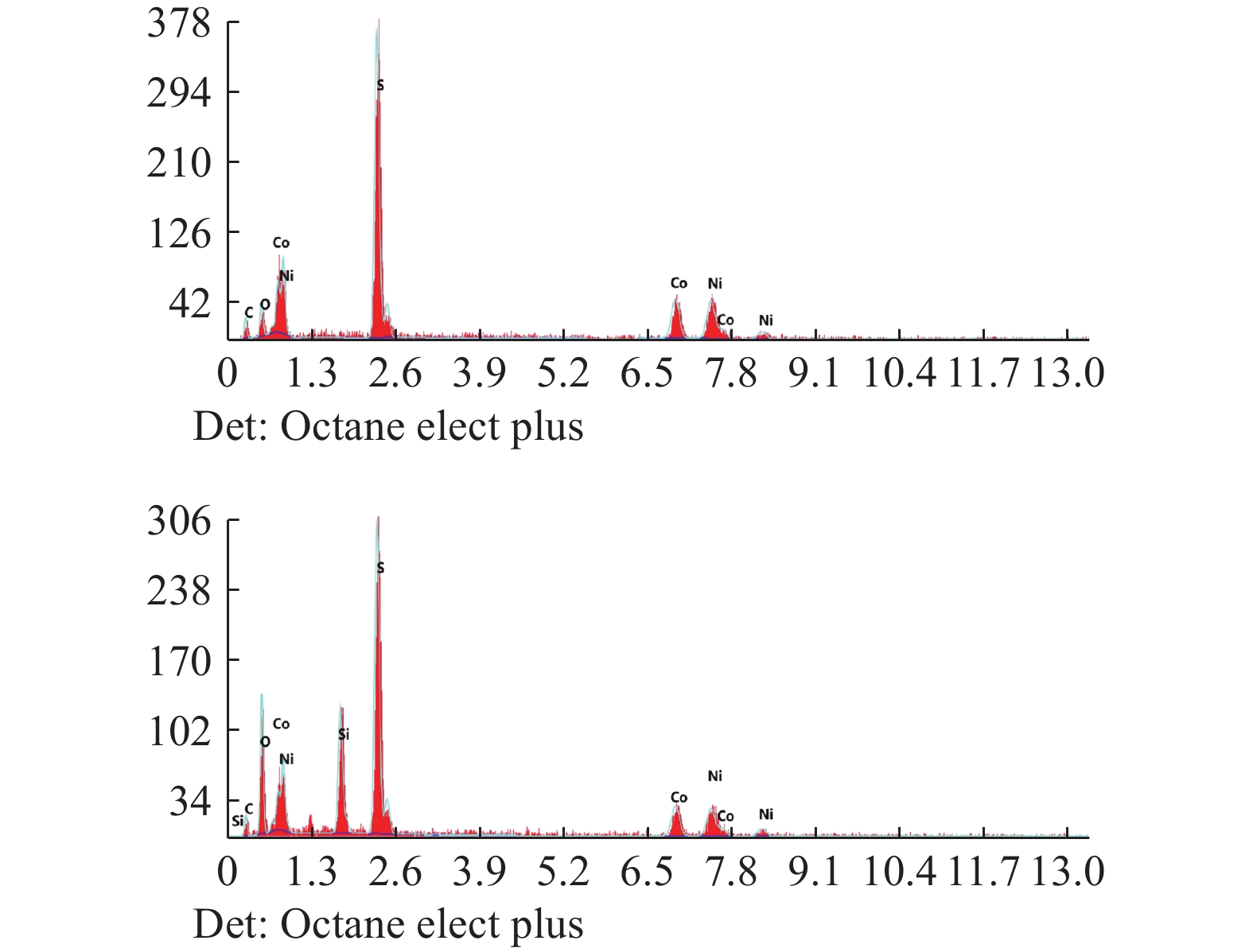
表 1 氢氧化钴中间品和硫化镍钴中间品主要化学成分
Table 1. Main chemical composition of cobalt hydroxide intermediate and nickel-cobalt sulfide intermediate
干基/% 水分
/%Ni Co Mn Fe Cu Si 氢氧化钴 0.32 25.45 8.54 1.81 0.36 0.69 33.74 硫化镍钴 30.61 16.97 <0.001 59.95 表 2 优化条件下实验结果
Table 2. Test results at optimum conditions
序号 浸出液 浸出渣 钴中间品
Mn浸出率
/%NiS利
用率
/%pH值 金属合量/(mol/L) 渣率
/%Ni
/%Co
/%Mn
/%1 1.36 1.98 3.22 9.83 9.45 0.10 99.93 90.63 2 1.30 1.99 2.87 8.35 7.43 0.12 99.93 88.69 3 1.27 2.04 3.18 7.82 8.33 0.13 99.91 92.64 平均 — — 3.09 — — — 99.92 90.65 -
[1] 陈忠玉, 刘强, 江皇义, 等. 分级-反浮选-重选高效分选刚果(金)某氧化钴铜矿[J]. 矿产综合利用, 2021(4):170-175.CHEN Z Y, LIU Q, JIANG H Y, et al. Beneficiation treatment of a cobalt-copper mine in DRC by combined process of classification-reverse flotation-gravity separation[J]. Multipurpose Utilization of Mineral Resources, 2021(4):170-175.
CHEN Z Y, LIU Q, JIANG H Y, et al. Beneficiation treatment of a cobalt-copper mine in DRC by combined process of classification-reverse flotation-gravity separation[J]. Multipurpose Utilization of Mineral Resources, 2021(4):170-175.
[2] Quentin Dehaine, Laurens T Tijsseling, Hylke J Glass. Geometallurgy of cobalt ores: A review[J]. Minerals Engineering, 2021, 160(1):106656.
[3] F. K. Crundwell, N. B. du Preez, B. D. H. Knights. Production of cobalt from copper-cobalt ores on the African Copperbelt-an overview[J]. Minerals Engineering, 2020, 156(1):106450.
[4] 张兴勋. 从某萃余液除杂后液中回收钴的实验研究[J]. 矿产综合利用, 2020(2):151-155.ZHANG X X. Study on precipitation of cobalt from purified raffinate[J]. Multipurpose Utilization of Mineral Resources, 2020(2):151-155.
ZHANG X X. Study on precipitation of cobalt from purified raffinate[J]. Multipurpose Utilization of Mineral Resources, 2020(2):151-155.
[5] 熊以俊, 陈斌, 谢欣旭. 亚铁离子加氧浸出钴中间品的钴[J]. 有色金属(冶炼部分), 2022(1):14-19.XIONG Y J, CHEN B, XIE X X. Oxygen leaching of cobalt intermediate by ferrous ions[J]. Nonferrous Metals (Extractive Metallurgy), 2022(1):14-19.
XIONG Y J, CHEN B, XIE X X. Oxygen leaching of cobalt intermediate by ferrous ions[J]. Nonferrous Metals (Extractive Metallurgy), 2022(1):14-19.
[6] 梁伟华, 郑世林, 付海阔. 双氧水还原浸出非洲氧化铜钴矿的试验研究[J]. 有色金属材料与工程, 2018, 39(4):38-41.LIANG W H, ZHENG S L, FU H K. Experimental Study on Reducing Leaching of African Copper-cobalt Oxide Ore by Hydrogen Peroxide[J]. Nonferrous Metal Materials And Engineering, 2018, 39(4):38-41. doi: 10.3969/j.issn.1006-0308.2001.02.008
LIANG W H, ZHENG S L, FU H K. Experimental Study on Reducing Leaching of African Copper-cobalt Oxide Ore by Hydrogen Peroxide[J]. Nonferrous Metal Materials And Engineering, 2018, 39(4):38-41. doi: 10.3969/j.issn.1006-0308.2001.02.008
[7] 谢洪珍. 还原浸出—除杂—活性氧化镁沉淀工艺从刚果金某氧化铜钴矿中回收钴[J]. 矿产保护与利用, 2021, 41(05):50-54.XIE H Z. Study on recovering cobalt from the Comgo cobalt-containing copper oxide ore by reduction leaching-Impurities removal-cobalt precitation with active magnesium oxide[J]. Conservation and Utilization of Mineral Resources, 2021, 41(05):50-54. doi: 10.3969/j.issn.1002-5065.2019.10.090
XIE H Z. Study on recovering cobalt from the Comgo cobalt-containing copper oxide ore by reduction leaching-Impurities removal-cobalt precitation with active magnesium oxide[J]. Conservation and Utilization of Mineral Resources, 2021, 41(05):50-54. doi: 10.3969/j.issn.1002-5065.2019.10.090
[8] 钟斌, 曾清全. 硫化沉淀法回收镍镁液中的镍[J]. 有色金属科学与工程, 2015, 6(2):53-56.ZHONG B, ZENG Q Q. Recovery of nickel from nickel-magnesium liquids by sulfide precipitation[J]. Nonferrous Metal Science and Engineering, 2015, 6(2):53-56.
ZHONG B, ZENG Q Q. Recovery of nickel from nickel-magnesium liquids by sulfide precipitation[J]. Nonferrous Metal Science and Engineering, 2015, 6(2):53-56.
-



 下载:
下载: