Study on Removal of Cobal and Nickel from Manganese Sulfate Leaching Solution by Carbonization Method
-
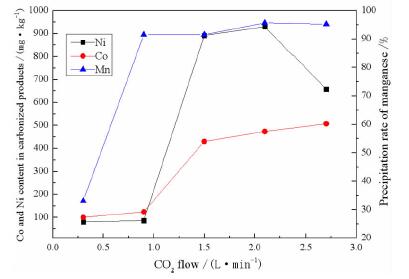
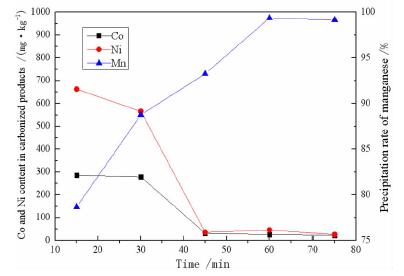
摘要: 采用碳化法去除硫酸锰溶液中的钴、镍离子。以CO2为碳化剂,NaOH为pH调节剂,将硫酸锰溶液中的Mn2+以碳酸锰沉淀的形式从原溶液中分离出来,然后用硫酸将沉淀物溶解,从而达到除杂的目的。考察CO2流量、反应温度、pH值及反应时间对钴、镍离子去除效果的影响。结果表明,反应温度25℃,溶液pH值为7.5,二氧化碳流量为0.9 L/min,反应时间为60 min时最为适宜,此时碳酸锰产品中钴、镍元素含量比硫酸锰一次结晶产物分别降低了0.031 29%和0.088 5%,其含量分别为0.003%和0.005%,符合高纯碳酸锰GB10503-89 I型品的标准。Abstract: The carbonization method was used to remove cobalt and nickel ions from the manganese sulfate solution. Mn2+ in the manganese sulfate solution was separated from the original solution in the form of manganese carbonate precipitation with the carbonizing agent of CO2, pH adjuster of NaOH. Then the precipitate was dissolved with sulfuric acid to remove impurities. The effects of CO2 flow rate, reaction temperature, pH value and reaction time on the removal of cobalt and nickel ions were investigated. The optimal experimental conditions with the reaction temperature of 25 ℃, the solution pH of 7.5, the carbon dioxide flow rate of 0.9 L/min, and the reaction time of 60 min were adopted. Under these conditions, the content of cobalt and nickel in the carbonized product was reduced by 0.03129% and 0.0885% compared with the primary crystallization product of manganese carbonate. The remaining amounts were 0.003% and 0.005%, respectively, which was in line with the standard of high-purity manganese carbonate GB 10503-89 type I product.
-
Key words:
- carbonized method /
- manganese sulfate solution /
- cobalt-nickel /
- deep impurity removal
-

-
表 1 样品元素分析结果
(mg·L -1) Table 1. Elemental analysis results of manganese sulfate leaching solution
Element Content Element Content Element Content Mn 5.04×104 As 1.05 Cd 1.15 Co 12.2 Cr 0.35 Mo 51.3 Ni 30.3 V 0 Sb 0 Mg 628.4 Al 5.6 Ti 0.15 Ca 750 Fe 2.75 Na 93.5 Cu 2 K 32.95 表 2 硫酸锰结晶杂质ICP成分分析
Table 2. ICP analysis of manganese sulfate crystal impurity
Element Fe Co Ni Mg Ca Na Cu K Al Ti Cd Content /(mg·kg-1) 7.5 342.9 935 7379 1263 1021 7.2 75.6 44 1.2 0.9 Quality score /% 0.000 75 0.034 29 0.093 50 0.700 00 379.000 00 0.102 10 0.000 72 0.007 56 0.004 40 0.000 12 0.000 09 表 3 25 ℃理论沉淀pH
Table 3. Theoretical precipitate pH at 35 ℃
Precipitation MnCO3 Mn(OH)2 CoCO3 NiCO3 pH Start 3.7 8.6 7.6 7.7 pH End 6.9 10.1 9.2 9.1 表 4 检测仪器
Table 4. Testing equipment
仪器名称 仪器型号 仪器生产商 ICP-MS质谱仪 Optima-8300 美国PE公司 XRD衍射仪 X’Pert PRO MPD 荷兰帕纳科有限公司 表 5 产物元素含量
Table 5. Product element content
Element Ca Mg Co Ni Content/% 0.03 0.01 0.003 0.005 表 6 高纯碳酸锰质量指标
Table 6. High purity manganese carbonate quality index
Element GB10503-89 I This method Ca ≤0.03% 0.03% Mg ≤0.02% 0.01% Co ≤0.005% 0.003% Ni ≤0.005% 0.005% -
[1] 郁先哲, 李能学, 宋金奎, 等.电解锰工业生产中硫酸锰的净化处理研究[J].无机盐工业, 2017, 49(6):50-52. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wjygy201706012
[2] 黄学杰.锂离子电池及相关材料进展[J].中国材料进展, 2010, 29(8):46-52, 36. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgcljz201008007
[3] 岳林.高磷菱锰矿焙烧特性及焙烧—氨浸工艺研究[D].重庆: 重庆大学, 2016.
[4] 陈丽鹃, 刘大为, 彭天剑, 等.硫酸锰溶液净化工艺研究[J].企业技术开发, 2012, 31(Z1):128-129. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=qyjskf201202060
[5] 刘京, 武佳, 冯江涛, 等.硫酸锰制备及净化研究进展[J].中国锰业, 2017, 35(5):114-118. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgmengy201705031
[6] 陈飞宇, 吴烽.高纯硫酸锰制备中除重金属新工艺的研究[J].中国锰业, 2012, 30(2):26-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgmengy201202008
[7] 宋玄, 李裕.碳酸镍的生产方法[J].无机盐工业, 2014, 46(1):55. http://d.wanfangdata.com.cn/Patent/CN201280015200.3/
[8] 张志华, 赵海涛.碳酸钠沉淀法合成高纯碱式碳酸镍的热力学分析[J].金属材料与冶金工程, 2013, 41(2), 22-25. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hnyj201302006
[9] 韩笑, 朱继俊, 孟凡兵, 刘玉珠.一种含锰废液制取高纯硫酸锰的方法: CN104445424A[P].2015-03-25.
[10] 李昌新, 李秋月, 喻源, 钟宏, 王帅.以高硫锰矿制备电池用硫酸锰的净化除杂工艺研究[J].无机盐工业, 2018, 50(7):27-32. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wjygy201807006
[11] ALLEN RW. An improved method of removing dissolved ferric iron from iron-bearing solution: Australian Patent424, 095[P]. 1970-05-15.
[12] AHMAD B J, CHENG WH, LOW W M, et al. Study on the removal of iron and manganese in groundwater by granular activated carbon [J]. Desalination, 2005, 182(1): 347-353. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2fe546acc2fd44f7c1ea4496596e0b40
[13] 高昭伟.二氧化锰深度净化硫酸锰溶液中钼的行为及机理研究[D].贵阳: 贵州大学, 2019.
-



 下载:
下载: