Recovery Germanium by Microwave Alkaline Roasting-water Dissolving from Zinc Oxide Dust Bearing Germanium
-
摘要:
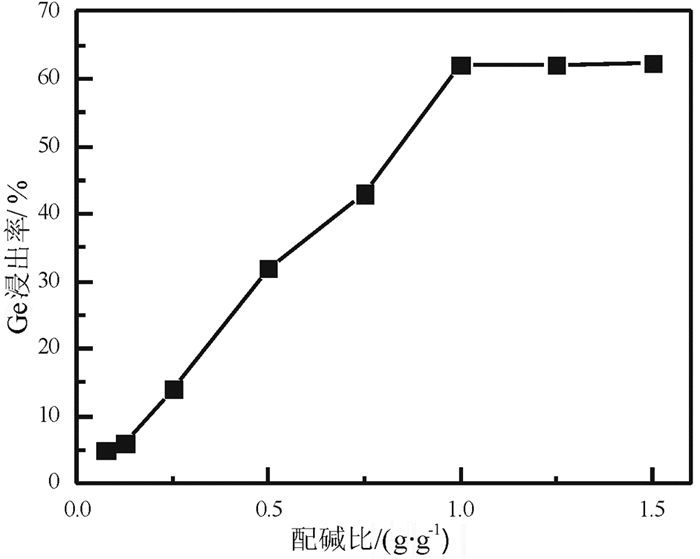
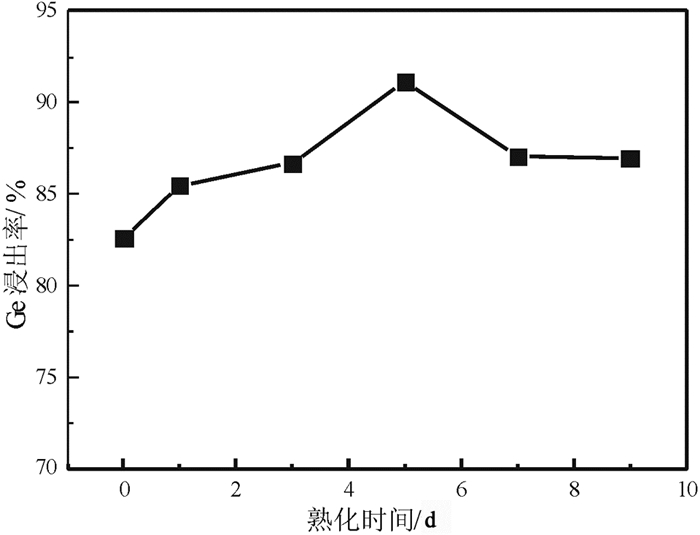
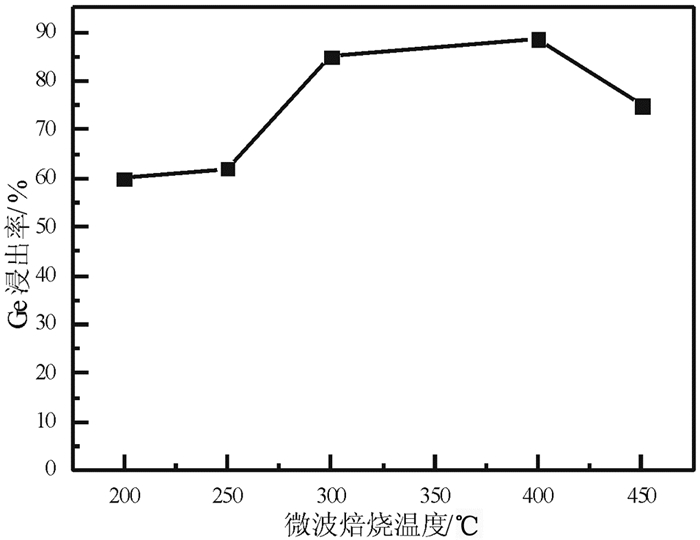
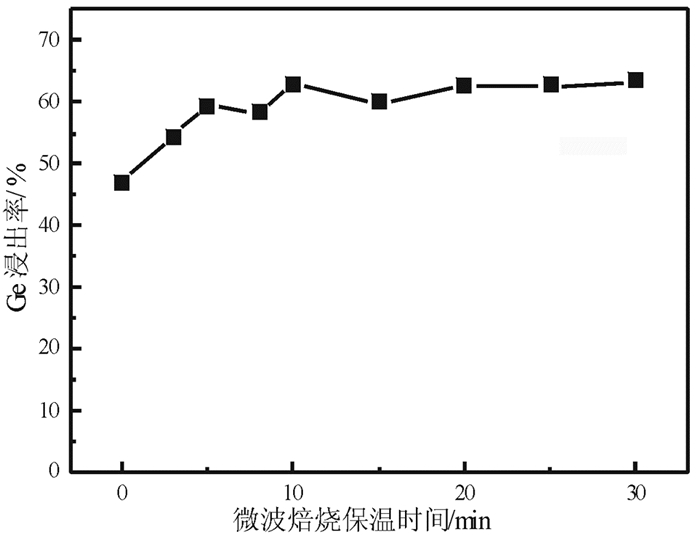
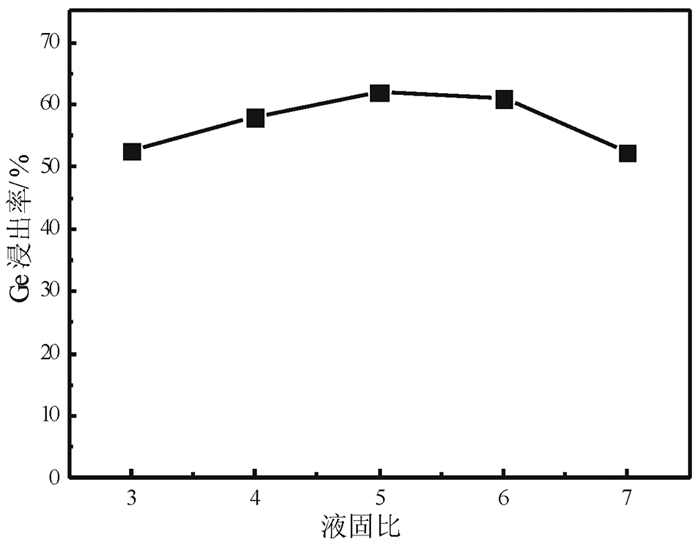
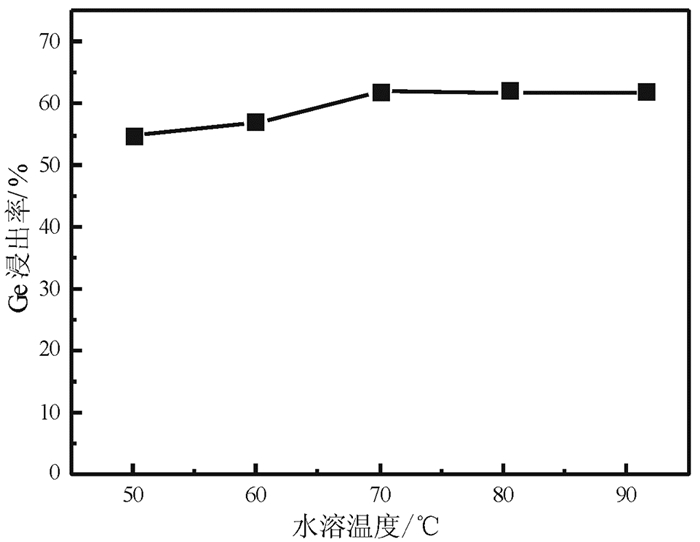
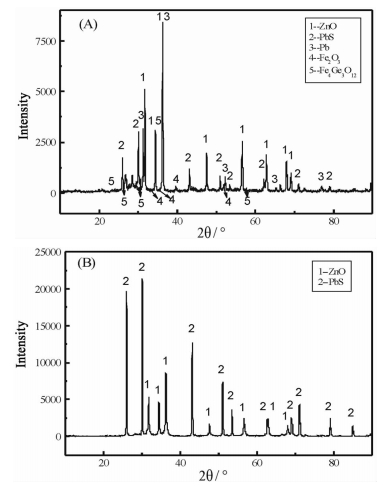
提出了微波碱性焙烧—水溶含锗氧化锌烟尘的新工艺,研究了配碱比、熟化时间、微波焙烧温度、液固比、水溶温度等对锗浸出率的影响规律。结果表明:在配碱比1 g·g-1、熟化时间5 d、微波焙烧温度400 ℃、保温时间10 min、液固比5 mL/g和水溶温度70 ℃时,锗的最佳浸出率为91.15%,与现有的常规碱性焙烧含锗氧化锌烟尘工艺对比可知,碱性焙烧温度从950~1 100 ℃降低至400 ℃,碱性焙烧保温时间由1~4 h降低至10 min,锗浸出率由80.35%提高至91.15%。
Abstract:Microwave alkaline roasting-water dissolving process was proposed to improve the germanium extraction. Alkali-material ratio, microwave heating temperature and leaching temperature are the significant factors for the process. By analyzing each factors systematically, the optimized conditions were obtained as follows, alkali-material ratio of 1 g·g-1, aging time of 5 day, microwave heating at 400℃, microwave alkaline roasting holding time of 10 min, liquid-solid ratio of 5 mL/g, leaching temperature at 70℃ with the germanium extraction about 91.15%. Compared with the existing methods, the alkaline roasting temperature was reduced from 950~1 100℃ to 408℃ and the alkaline roasting holding time was greatly reduced from 1~4 h to 10 min.
-
Key words:
- zinc oxide dust /
- germanium /
- leaching /
- microwave roasting /
- alkaline roasting
-

-
表 1 含锗氧化锌烟尘原料的化学成分 /%
Table 1. Major chemical ingredients of zinc oxide dust bearing germanium
元素 Zn Pb Cu As Cd Fe Sb Al Bi Ge Ca Ag Cl In K S 含量 51.50 20.50 0.14 1.04 0.52 0.44 0.27 0.17 0.06 510 0.04 0.04 0.04 0.02 0.13 3.00 注:Ge含量单位为g/t。 -
[1] Depuydt Ben, Theuwis Antoon, Romandic Igor. Germanium: from the first application of czochralski crystal growth to large diameter dislocation-free wafers[J]. Materials Science in Semiconductor Processing, 2006, 9(4-5): 437-443. doi: 10.1016/j.mssp.2006.08.002
[2] Thostenson E T, Chou T W. Microwave processing: fundamentals and applications[J]. Composites Part A: Applied Science and Manufacturing, 1999, 30(9): 1055-1071. doi: 10.1016/S1359-835X(99)00020-2
[3] Dutrizac J E, Chen T T, Longton R J. The mineralogical deportment of germanium in the clarksville electrolytic zinc plant of savage zinc inc[J]. Metallurgical and Materials Transactions B, 1996, 27(4): 567. doi: 10.1007/BF02915654
[4] 李琛. 韶冶密闭鼓风炉熔炼过程中锗铟的富集与综合回收[D]. 长沙: 中南大学, 2004.
http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y672949 [5] Zhang Libo, Guo Wenqian, Peng Jinhui, et al. Comparison of ultrasonic-assisted and regular leaching of germanium from by-product of zinc metallurgy[J]. Ultrasonics Sonochemistry, 2016, 31: 143-149. doi: 10.1016/j.ultsonch.2015.12.006
[6] Nusen Sankum, Zhu Zhaowu, Chairuangsri Torranin, et al. Recovery of germanium from synthetic leach solution of zinc refinery residues by synergistic solvent extraction using lix 63 and ionquest 801[J]. Hydrometallurgy, 2015, 151:122-132. doi: 10.1016/j.hydromet.2014.11.016
[7] Liu Fupeng, Liu Zhihong, Li Yuhu, et al. Recovery and separation of gallium(Ⅲ) and germanium(Ⅳ) from zinc refinery residues: Part Ⅰ: Leaching and iron(Ⅲ) Removal[J]. Hydrometallurgy, 2017, 171: 149-156. doi: 10.1016/j.hydromet.2017.05.009
[8] Liu Fupeng, Liu Zhihong, Li Yuhu, et al. Extraction of gallium and germanium from zinc refinery residues by pressure acid leaching[J]. Hydrometallurgy, 2016, 164: 313-320. doi: 10.1016/j.hydromet.2016.06.006
[9] Liang Duoqiang, Wang Jikun, Wang Yunhua. Difference in dissolution between germanium and zinc during the oxidative pressure leaching of sphalerite[J]. Hydrometallurgy, 2009, 95(1-2): 5-7. doi: 10.1016/j.hydromet.2008.03.005
[10] 王娜. 石煤矿提钒绿色工艺的基础研究[D]. 重庆: 重庆大学, 2010.
http://cdmd.cnki.com.cn/article/cdmd-10611-2010216658.htm [11] 彭金辉, 郭胜惠, 张世敏, 等.微波加热干燥钛精矿研究[J].昆明理工大学学报(理工版), 2004(4): 5-9. http://doi.wanfangdata.com.cn/10.3969/j.issn.1007-855X.2004.04.002
[12] 付润泽. 微波辅助磨细惠民铁矿实验研究[D]. 昆明: 昆明理工大学, 2011.
http://cdmd.cnki.com.cn/Article/CDMD-10674-1012263253.htm [13] Ali A Y, Bradshaw S M. Quantifying damage around grain boundaries in microwave treated ores[J]. Chemical Engineering and Processing: Process Intensification, 2009, 48(11-12): 1566-1573. doi: 10.1016/j.cep.2009.09.001
[14] Yang Kun, Li Shiwei, Zhang Libo, et al. Microwave roasting and leaching of an oxide-sulphide zinc ore[J]. Hydrometallurgy, 2016, 166: 243-251. doi: 10.1016/j.hydromet.2016.07.012
-




 下载:
下载: