STUDY ON THE ADSORBABILITY OF OIL SHALE ON COBALT IONS
-
摘要:
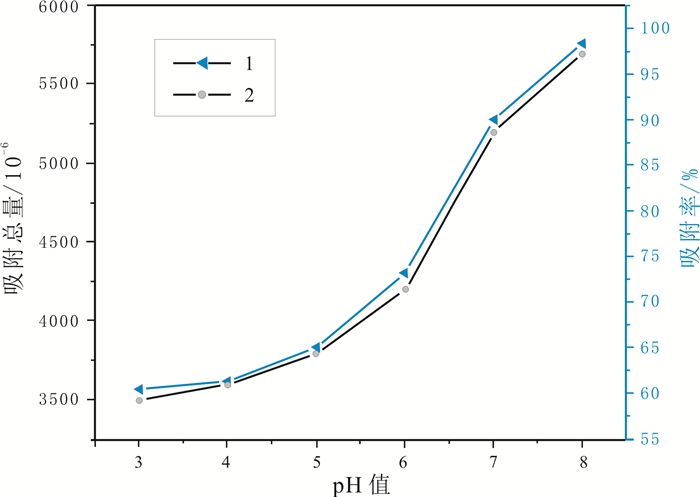
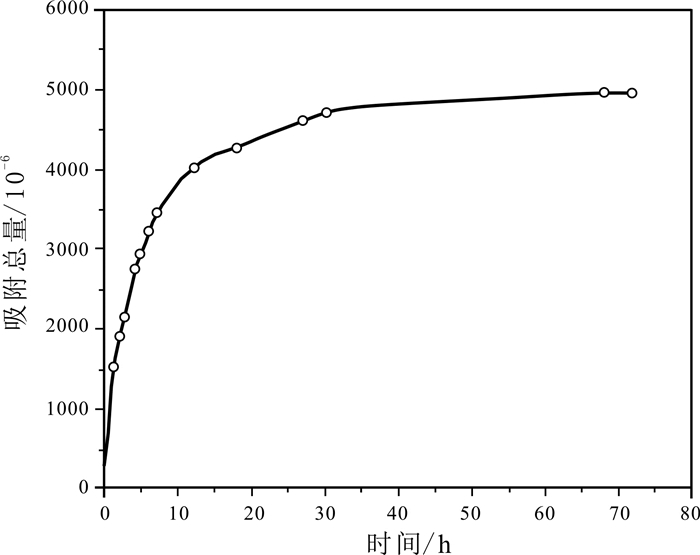
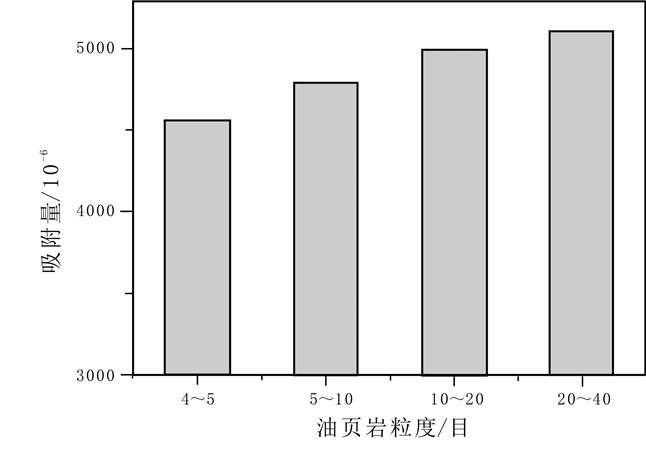
油页岩中因含有大量的黏土矿物而对金属离子具有一定的吸附能力.采用静态吸附法对油页岩吸附钴离子的影响因素及吸附动力学进行了研究.结果表明,油页岩粒度、溶液浓度、溶液pH值、吸附时间等均对吸附性能有一定影响.油页岩对钴离子的吸附量随样品粒径的减小而增大;随着钴离子初始浓度的增加,油页岩对钴离子的吸附总量增加;溶液pH值在3~8范围内,油页岩对钴离子的吸附量和吸附率随着pH值的增大呈上升趋势.通过吸附动力学研究发现,油页岩对钴离子的吸附过程符合准二级动力学过程和粒子内扩散机理.
Abstract:Oil shale contains a large amount of clay minerals and thus has a certain adsorption capacity for metal ions. The influence factors and adsorption kinetics of oil shale adsorption on cobalt ions(Co2+) are studied by static adsorption method. The results show that the oil shale granularity, solution concentration, pH value and absorption time all have certain effect on the adsorption capability. The adsorption amount of Co2+ by oil shale increases with the decrease of sample grain size. With the increase of initial concentration of cobalt ions, the total amount of adsorbed Co2+ also increases. With the solution pH ranging from 3 to 8, the adsorption amount and rate of Co2+ increase with the increase of pH value. According to the adsorption kinetics, the adsorption process of Co2+ conforms to the pseudo-second-order kinetic process and intraparticle diffusion mechanism.
-
Key words:
- oil shale /
- adsorbability /
- cobalt ions (Co2+)
-

-
表 1 初始浓度对油页岩吸附Co2+离子的影响
Table 1. Effect of Co2+ initial concentration on the adsorbability of oil shale
初始浓度/(mg/L) 平衡浓度/(mg/L) 平衡吸附量/(mg/L) 吸附总量/10-6 吸附率/% 82.1 24.7 57.4 2870 69.9 125.8 46.4 79.4 3972 63.1 187.5 90.2 97.3 4863 51.9 240.5 137.7 102.8 5140 42.7 302.5 199.3 103.2 5160 34.1 345.9 240.6 105.3 5264 30.4 表 2 油页岩对Co2+的吸附动力学常数
Table 2. Kinetic constants of oil shale adsorption on Co2+
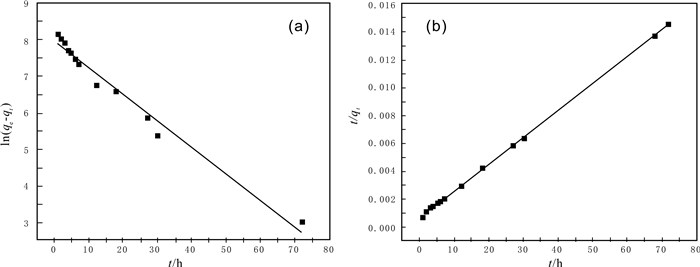
模型 参数 Co2+ 准一级动力学 R2 0.9749 K1(h-1) 0.0725 qe,cal(10-6) 2824.4 准二级动力学 R2 0.9986 K2(10-11 h-1) 5.48 qe,cal(10-6) 5220 粒子内扩散 R2 0.9776 Ki(10-6 h-1/2) 1112.5 C(10-6) 413.0 -
[1] 钱家麟, 王剑秋, 李术元.世界油页岩资源利用和发展趋势[J].吉林大学学报(地球科学版), 2006, 36(6):877-887. http://d.old.wanfangdata.com.cn/Periodical/cckjdxxb200606003
[2] Wilson K, Yang H, Seo C W, et al. Select metal adsorption by activated carbon made from peanut shells[J]. Bioresource Technology, 2006, 97(18):2266-2270. doi: 10.1016/j.biortech.2005.10.043
[3] 钱家麟, 尹亮.油页岩:石油的补充能源[M].北京:中国石化出版社, 2008.
[4] Fan C, Yan J W, Huang Y R, et al. XRD and TG-FTIR study of the effect of mineral matrix on the pyrolysis and combustion of organic matter in shale char[J]. Fuel, 2015, 139:502-510. doi: 10.1016/j.fuel.2014.09.021
[5] 王擎, 张宏喜, 迟铭书, 等.矿物质对桦甸油页岩热解产物影响特性[J].燃料化学学报, 2016, 44(3):328-334. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201603010
[6] Xu D, Tan X L, Chen C L, et al. Adsorption of Pb (Ⅱ) from aqueous solution to MX-80 bentonite:Effect of pH, ionic strength, foreign ions and temperature[J]. Applied Clay Science, 2008, 41(1/2):37-46. https://www.researchgate.net/publication/222538193_Adsorption_of_PbII_from_Aqueous_Solution_to_MX-80_Bentonite_Effect_of_pH_Ionic_Strength_Foreign_Ions_and_Temperature
[7] 曹积飞, 杨秋荣, 李英杰, 等.粘土矿物对重金属有害元素吸附性研究[J].环境科学与技术, 2008, 31(1):42-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkxyjs200801012
[8] Veli S, Alyüz B. Adsorption of copper and zinc from aqueous solutions by using natural clay[J]. Journal of Hazardous Materials, 2007, 149(1):226-233. doi: 10.1016/j.jhazmat.2007.04.109
[9] Tahir S S, Rauf N. Thermodynamic studies of Ni (Ⅱ) adsorption onto bentonite from aqueous solution[J]. The Journal of Chemical Thermodynamics, 2003, 35(12):2003-2009. doi: 10.1016/S0021-9614(03)00153-8
[10] Al-Asheh S, Banat F. Adsorption of copper and zinc by oil shale[J]. Environmental Geology, 2001, 40(6):693-698. doi: 10.1007/s002540000234
[11] Jiang H F, Song L H, Cheng Z Q, et al. Influence of pyrolysis condition and transition metal salt on the product yield and characterization via Huadian oil shale pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2015, 112:230-236. doi: 10.1016/j.jaap.2015.01.020
[12] 李俊锋, 宋丽华, 张永强, 等.一种用于油页岩提取页岩油的催化剂及其使用方法: 中国, 103464179A[P]. 2013-09-24.
[13] 宋丽华.过渡金属盐对油页岩热解催化作用研究[D].长春: 吉林大学, 2016: 1-127.
http://cdmd.cnki.com.cn/Article/CDMD-10183-1017016042.htm [14] 张永强.提高油页岩中页岩油提取率方法研究[D].长春: 吉林大学, 2014: 1-51.
http://cdmd.cnki.com.cn/Article/CDMD-10183-1014268371.htm [15] Ahmad M A, Alrozi R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon:Equilibrium, kinetic and thermodynamic studies[J]. Chemical Engineering Journal, 2011, 171(2):510-516. doi: 10.1016/j.cej.2011.04.018
[16] Pei Y Y, Liu J Y. Adsorption of Pb2+ in wastewater using adsorbent derived from grapefruit peel[J]. Advanced Materials Research, 2011, 391-392:968-972. doi: 10.4028/www.scientific.net/AMR.391-392.968
[17] Abu-El-Sha'r W Y, Gharaibeh S H, Al-Kofahi M M. Removal of selected heavy metals from aqueous solutions using a solid by-product from the Jordanian oil shale refining[J]. Environmental Geology, 1999, 39(2):113-116. doi: 10.1007/s002540050441
[18] Ngah W S W, Hanafiah M A K M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents:A review[J]. Bioresource Technology, 2008, 99(10):3935-3948. doi: 10.1016/j.biortech.2007.06.011
[19] Chiou M S, Li H Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads[J]. Chemosphere, 2003, 50(8):1095-1105. doi: 10.1016/S0045-6535(02)00636-7
[20] Wang Y, Mu Y, Zhao Q B, et al. Isotherms, kinetics and thermodynamics of dye biosorption by anaerobic sludge[J]. Separation and Purification Technology, 2006, 50(1):1-7. doi: 10.1016/j.seppur.2005.10.012
[21] Ngah W S W, Fatinathan S. Adsorption of Cu (Ⅱ) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads[J]. Chemical Engineering Journal, 2008, 143(1/3):62-72. https://www.sciencedirect.com/science/article/pii/S1385894707008005
[22] 孙小莉, 曾庆轩, 冯长根.多胺型阴离子交换纤维吸附铬(Ⅵ)的动力学[J].物理化学学报, 2009, 25(10):1951-1957. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb200910002
-



 下载:
下载: