Data Normalization and Quality Control of Light Element Stable Isotope Analyses by Means of Continuous Flow Isotope Ratio Mass Spectrometry
-
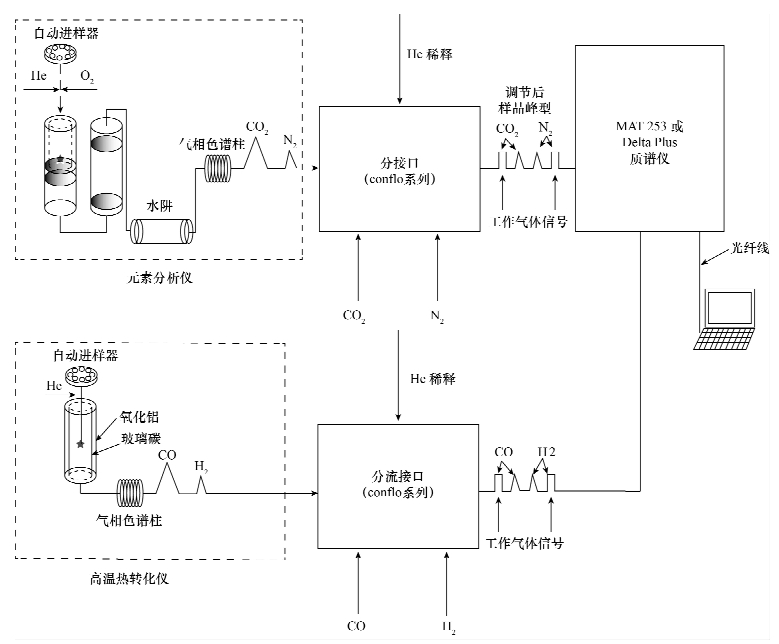
摘要: 同位素比值质谱分析方法是准确测量各种同位素相对丰度的标准方法。连续流同位素质谱的出现不仅提高运行效率,也降低了样品用量并提高灵敏度。但是,要使这种方法获得更好准确度和精度的同位素数据,并做到所获得数据可与其他实验室结果进行类比,从而得到可靠的同位素数据,这就需要好的分析策略和运行方案,还需要对仪器日常性能和数据质量进行严密的监视管控,而且还取决于原始数据如何进一步标准化到国际同位素尺度上。因此,同位素比值质谱结合元素分析仪(或热转换元素分析仪)连续流方法要实现可靠的稳定同位素分析需要:①设备安装和环境控制、测试准备、样品制备和称量、标准物质选择及序列等规范化质量控制措施;②严格校准仪器系统(包括调节灵敏度和线性,背景值监测,稳定性检测,H3+系数校正等);③可靠的数据处理。目前不同的实验室,采用标准物质来标定系统、对测量的同位素数据进行标准化,以及利用控制曲线来监测系统稳定性并对不确定度的计算,这些策略往往都不同。因此,统一的数据处理方案是被高度期待的。目前最好的执行方案是基于线性回归的两点或多点标准化方法。如果每一批样品中测量两个不同的标准物质四次,或者测量四个标准物质两次,那么不确定度会降低50%。当前同位素比值质谱能够测定同位素比值的不确定度一般要好于0.02‰。但是,标准物质的使用既要考虑样品的性质,同时要涵盖它们未知同位素组成的范围,尤其氢同位素在现阶段缺乏标准物质和测量的仪器精度较差(比碳、氮、氧等要低一个数量级)的情况下,这显然是稳定同位素分析者的一个重大挑战。本文概括了同位素比值质谱结合元素分析仪(或热转换元素分析仪)的基本操作原理和分析实践,将数据处理运用到同位素比值分析之中,获得连续流同位素比值质谱分析结果的合理准确度和精度。Abstract: The technique of Isotope Ratio Mass Spectrometry(IRMS)is the gold standard of accurate and precise analyses for all sorts of isotopic relative abundance amongst analytical techniques. In many fields, the extensive application of continuous flow IRMS not only improves effective runs, but also reduces sample size as well as enhancing sensitivity. However, for the purpose of obtaining more accurate results, which can be compared between different laboratories, it is imperative to have good strategies and protocols in the run, monitoring of routine analytical performance and quality. In addition, detailed calibration for the raw data is highly desirable. In order to obtain reliable data by using IRMS coupled with EA (TC/EA), requirements need to be met as follows: (1) instrument set-up and environmental control, measurement preparation, sample preparation and weight, reference materials utilization and selection, sequence etc; (2) strictly calibrated systems including tuning sensitivity and linearity, monitoring the background, stability detection, H3+ correction; (3) reliable data reduction. The strategies of calibrating, normalization and control linear by using reference materials are discriminated in different labs. So it is expected to have a unified data-processing programme. Two-point normalization (or multi-point normalization) is the best executive program at present. If two different reference materials are used four times for normalization, or four different reference materials are used two times for each batch of samples, this may reduce the normalization uncertainty by 50%. The modern IRMS is capable of measuring natural isotope ratio variations with an uncertainty better than 0.02. Nevertheless, it is a challenge to the use of reference materials with the cover range of unknown isotopes, especially for H isotope absence of the standard materials and poor precision (an order of magnitude lower compared with carbon, nitrogen, oxygen isotope). This paper aims to highlight general principles of IRMS coupled to an EA or a TC/EA and the practices of analytical application of stable isotope ratio measurement. The knowledge on the protocols of continuous flow IRMS analyses is necessary for the acquisition of reasonable accuracy and precision for stable isotope composition.
-

-
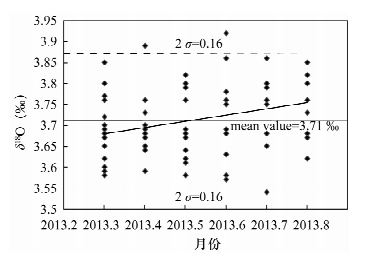
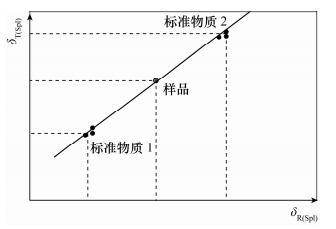
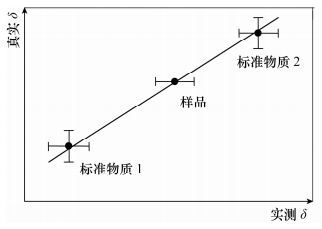
图 4 样品的合成不确定度示意图(据文献[2]修改)
Figure 4.
表 1 连续流系统条件下的样品和标准物质序列的典型构成(以碳酸盐13C同位素为例)
Table 1. The classical sample and reference materials batch sequence compositions in continuous flow experimental conditions (13C isotope analysis of carbonate as an example)
样品编号 δ值 δT值(‰) 测量值 平均值 σ 空白(锡杯或银杯) ** ** ** ** 标准物质1 (L-SVEC) -46.56 -46.56 0.13 -46.48 标准物质1 (L-SVEC) -46.73 标准物质1 (L-SVEC) -46.40 标准物质1 (L-SVEC) -46.55 样品1 ** ** ** ** 样品1 ** 样品1 ** … ** ** ** ** 空白(锡杯或银杯) ** ** ** ** 监测标准(USGS24) -16.023 -16.049
(IAEA推荐值)监测标准(USGS24) -16.078 -16.052 0.03 监测标准(USGS24) -16.056 样品n ** ** ** ** 样品n ** 样品n ** 标准物质2 (NBS19) 1.89 1.92 0.05 1.95 标准物质2 (NBS19) 1.98 标准物质2 (NBS19) 1.90 标准物质2 (NBS19) 1.88 空白(锡杯或银杯) ** ** ** ** 注:“**”表示具体的测量值和计算值。 表 2 使用合成标准不确定度来计算样品的不确定度
Table 2. Calculation of uncertainty of sample utilizing combined standard uncertainty
序号 A B C D E F G H 1 参数 δ13(‰) 不确定度 - - - - - 2 δT(VSMOW) 0.0 0.3 0.3(B2+C2) 0(B2) 0(B2) 0(B2) 0(B2) 3 δT(SLAP) -427.5 0.3 -427.5(B3) -427.2(B3+C3) -427.5(B3) -427.5(B3) -427.5(B3) 4 δR(VSMOW) 0.2 1.0 0.2(B4) 0.2(B4) 1.2(B4+C4) 0.2(B4) 0.2(B4) 5 δR(SLAP) -422.5 1.0 -422.5(B5) -422.5(B5) -422.5(B5) -421.5(B5+C5) -422.5(B5) 6 δR(SLAP) -120.0 1.2 -120.0(B6) -120.0(B6) -120.0(B6) -120.0(B6) -118.8(B5+C5) 7 - -121.4(根据方程(5)a 1.0(U(δT(Spl)=偏差和的平方根计算得出)偏差 -121.45(根据方程(5)a式计算) -121.28(根据方程(5)a式计算) -121.08(根据方程(5)a式计算) -121.65(根据方程(5)a式计算) -120.5(根据方程(5)a式计算) 8 - - - -0.05(D7-B7) 0.12(E7-B7) 0.32 (F7-B7) -0.25 (G7-B7) 0.9(H7-B7) 注:B2是指第B列和第2行交叉单元格。 -
[1] Midwood A J, McGaw B A.Recent developments in the analysis of light isotopes by continuous flow isotope ratio mass spectrometry[J].Analalytical Communication,1999,36:291-294. doi: 10.1039/a904908h
[2] Carter J F, Barwick V J.Good Practice Guide for Isotope Ratio Mass Spectrometry, FIRMS[M].ISBN 978-0-948926-31-0.2011.
[3] Brenna J T, Corso T N, Tobias H J, Caimi R J.High-precision continuous-flow isotope ratio mass spectrometry[J].Mass Spectrometry Review,1997,16:227-258. doi: 10.1002/(ISSN)1098-2787
[4] Midwood A J, McGaw B A.Recent developments in the analysis of light isotopes by continuous flow isotope ratio mass spectrometry[J].Analytical Communication,1999,36:291-294. doi: 10.1039/a904908h
[5] Révész K M, Landwehr J M.δ13C and δ18O isotopic composition of CaCO3 measured by continuous flow isotope ratio mass spectrometry: Statistical evaluation and verification by application to Devils Hole core DH-11 Calcite[J].Rapid communications in Mass Spectrometry,2002,16:2102-2114. doi: 10.1002/rcm.v16:22
[6] Spötl C, Vennemann T W.Continuous-flow isotope ratio mass spectrometric analysis of carbonate minerals[J].Rapid Communications in Mass Spectrometry,2003,17:1004-1006. doi: 10.1002/rcm.v17:9
[7] Fritzsche F, Tichomirowa M.Signal improvement in elemental analyzer/continuous flow isotope ratio mass spectrometry for samples with low sulfur content using a pre-concentration technique for on-line concentration adjustment[J].Rapid Communications in Mass Spectrometry,2006,20:1682-1697.
[8] Grassineau N V,Mattey D P, Lowry D.Sulfur isotope analysis of sulfide and sulfate minerals by continuous flow-isotope ratio mass spectrometry[J].Analytical Chemistry,2001,73(2):220-225. doi: 10.1021/ac000550f
[9] Grassineau N V.High-precision EA-IRMS analysis of S and C isotopes in geological materials[J].Applied Geochemistry,2006,21:756-765. doi: 10.1016/j.apgeochem.2006.02.015
[10] Fry B, Silva S R, Kendall C, Anderson R K.Oxygen isotope corrections for online δ34S analysis[J].Rapid Communications in Mass Spectrometry,2002,16:854-858. doi: 10.1002/(ISSN)1097-0231
[11] Yun M, Mayer B, Taylor S W.δ34S measurement on organic materials by continuous flow isotope ratio mass spectrometry[J].Rapid Communications in Mass Spectrometry,2005,19:1429-1436. doi: 10.1002/(ISSN)1097-0231
[12] Fry B.Coupled N, C and S stable isotope measurements using a dual-column gas chromatography system[J].Rapid Communications in Mass Spectrometry,2007,21:750-756. doi: 10.1002/(ISSN)1097-0231
[13] Paul D, Skrzypek G, Forizs I.Normalization of meas-ured stable isotope composition to isotope reference scale—A review[J].Rapid Communications in Mass Spectrometry,2007,21:3006-3014. doi: 10.1002/(ISSN)1097-0231
[14] Skrzypek G, Paul D.δ13C analyses of calcium carbo-nate: Comparison between the Gasbench and elemental analyzer techniques[J].Rapid Communications in Mass Spectrometry,2006,20:2915-2920. doi: 10.1002/(ISSN)1097-0231
[15] Skrzypek G, Sadler R, Paul D.Error propagation in normalization of stable isotope data: A Monte Carlo analysis[J].Rapid Communications in Mass Spectrometry,2010,24(18):2697-2705. doi: 10.1002/rcm.4684
[16] Skrzypek G, Sadler R.A strategy for selection of ref-erence materials in stable oxygen isotope analyses of solid materials[J].Rapid Communications in Mass Spectrometry,2011,25:1625-1630. doi: 10.1002/rcm.5032
[17] Spötl C, Vennemann T W.Continuous-flow isotope ratio mass spectrometric analysis of carbonate minerals[J].Rapid Communications in Mass Spectrometry,2003,17:1004-1006. doi: 10.1002/rcm.v17:9
[18] Skrzypek G.Normalization procedures and reference mat-erial selection in stable HCNOS isotope analyses: A review[J].Analytical and Bioanalytical Chemistry,2013,405:2815-2823. doi: 10.1007/s00216-012-6517-2
[19] 王政,刘卫国,文启彬.土壤样品中的氮同位素组成的元素分析仪-同位素质谱分析方法[J].质谱学报,2005,26(2):71-75. http://www.cnki.com.cn/Article/CJFDTOTAL-ZPXB200502001.htm
[20] 曹建平,黄奕普,刘广山,陈敏,李鸿宾.海洋悬浮颗粒中氮同位素的EA-IRMS法测定[J].台湾海峡,2003,22(1):1-8. http://www.cnki.com.cn/Article/CJFDTOTAL-TWHX200301000.htm
[21] 崔杰华,祁彪,王颜红.植物样品中稳定碳同位素的EA-IRMS系统分析方法[J].质谱学报,2008,29(1):24-29. http://www.cnki.com.cn/Article/CJFDTOTAL-ZPXB200801007.htm
[22] 王旭,张福松,丁仲礼.EA-Conflo-IRMS联机系统的燃烧转化率漂移及其对氮碳同位素比值测定的影响[J].质谱学报,2006,27(2):104-109. http://www.cnki.com.cn/Article/CJFDTOTAL-ZPXB200602008.htm
[23] 储雪蕾.一种新的、快速的碳、氮、硫同位素测定手段——EA-IRMS连线分析技术[J].矿物岩石地球化学通报,1996,15(4):259-262. http://www.cnki.com.cn/Article/CJFDTOTAL-KYDH604.013.htm
[24] 郑永飞,龚冰,王峥荣.岩石中的碳同位素比值的EA-MS测定及其地球化学应用[J].地质论评,1999,45(5):529-538. http://www.cnki.com.cn/Article/CJFDTOTAL-DZLP199905014.htm
[25] 张媛媛,贺行良,孙书文,朱志刚.元素分析仪-同位素比值质谱仪测定海洋沉积物有机碳稳定同位素方法初探[J].岩矿测试,2012,31(4):627-631. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS201204015.htm
[26] 刘运德,甘义群,余婷婷,刘存富,周爱国.微量水氢氧同位素在线同时测试技术——热转换元素分析同位素比质谱法[J].岩矿测试,2010,29(6):643-647. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS201006004.htm
[27] 龚冰,陈仁旭,郑永飞.大别—苏鲁造山带超高压变质岩矿物水含量和氢同位素组成[J].科学通报,2013,58(22):2169-2174. http://www.cnki.com.cn/Article/CJFDTOTAL-KXTB201322009.htm
[28] Werner R A, Brand W A.Referencing strategies and techniques in stable isotope ratio analysis[J].Rapid Communications in Mass Spectrometry,2001,15:501-519. doi: 10.1002/(ISSN)1097-0231
[29] Preston T, Owens N J P.Interfacing and automatic elemental analyser with an isotope ratio mass spectrometer: The potential for fully automated total nitrogen and nitrogen-15 analysis[J].Analyst,1983,108:971-977. doi: 10.1039/an9830800971
[30] Glesemann A, Jäger H J, Norman A L, Krouse H R, Brand W A.On-line sulfur isotope determination using an element analyser coupled to a mass spectrometer[J].Analytical Chemistry,1994,66:2816-2819. doi: 10.1021/ac00090a005
[31] Fourel F, Martineau F, Lécuyer C, Kupka H J, Lange L, Ojeimi C, Seed M.18O/16O ratio measurement of inorganic and organic materials by elemental analysis-pyrolysis-isotope ratio mass spectrometry continuous flow techniques[J].Rapid Communications in Mass Spectrometry,2011,25:2691-2696. doi: 10.1002/rcm.5056
[32] Gentile N, Besson L, Pazos D, Delémont O, Esseiva P.On the use of IRMS in forensic science: Proposal for a methodological approach[J].Forensic Science International,2011,212:260-271. doi: 10.1016/j.forsciint.2011.07.003
[33] Finnigan Gasbench Ⅱ Operating Manual[Z].
[34] Nelson S T.Sample vial influence on the accuracy and precision of carbon and oxygen isotope ratio analysis in continuous flow mass spectrometric applications[J].Rapid Communications in Mass Spectrometry,2000,14:293-297. doi: 10.1002/(ISSN)1097-0231
[35] Zha X P, Zhao Y Y, Zheng Y F.An online method combining a Gasbench Ⅱ with continuous flow isotope ratio mass spectrometry to determine the content and isotopic compositions of minor amounts of carbonate in silicate rocks[J].Rapid Communications in Mass Spectrometry,2010,24:2217-2216. doi: 10.1002/rcm.v24:15
[36] Brand W A.Mass Spectrometry Handware for Analyzing Stable Isotope Ratios[M]//de Groot P A, eds.Handbook of Stable Isotope Analytical Techniques (Vo1.1).Oxford: Elsevier B V,2004:805-819.
[37] Kornfeld A, Horton T W, Yakir D, Turnbull M H.Correcting for nonlinearity effect of continuous flow isotope ratio mass spectrometry across a wide dynamic range[J].Rapid Communications in Mass Spectrometry,2012,26:460-468. doi: 10.1002/rcm.6120
[38] Muccio Z, Jackson G P.Isotope ratio mass spectrometry[J].Analyst,2009,134:213-222. doi: 10.1039/B808232D
[39] Gröning M.International Stable Isotope Reference Materials[M]//de Groot P A, eds.Handbook of Stable Isotope Analytical Techniques (Vo1.1).Oxford: Elsevier B V,2004:874-906.
[40] Bièvre P D, Laeter D, Peiser H S, Reed W P.Reference materials by isotope ratio mass spectrometry[J].Mass Spectrometry Review,1993,12:143-172. doi: 10.1002/(ISSN)1098-2787
[41] Coplen T B, Brand W A, Gehre M, Gröning M, Meijer H A J, Toman B, Verkouteren R M.New guidelines for δ13C measurement[J].Analytical Chemistry,2006,78:2439-2441. doi: 10.1021/ac052027c
[42] Coplen T B.Guidelines and recommended terms for expression of stable-isotope-ratio and gas ratio measurement results[J].Rapid Communications in Mass Spectrometry,2011,25:2538-2560. doi: 10.1002/rcm.5129
[43] 中国合格评定国家认可委员会.化学分析中的不确定度的评估指南[S].2006:6.
-



 下载:
下载: