Study on Migration of Lead and Admium in the Port Environment and Model building
-
摘要: 港口矿产品堆场的重金属溶出作为港口土壤、水体的重要污染源不容忽视。本文在天津港口7个有代表性的矿产品堆场采集土壤和水体样品,确定重金属的污染状况;应用微宇宙系统模拟Cd和Pb在港口沉积物和水体生态系统中的迁移行为,通过建立多介质逸度模型对微宇宙系统的模拟情况进行拟合,探索Cd和Pb在沉积物和水体中的归趋和迁移规律。现场采样分析表明,矿产品堆场重金属污染较为严重,其中Cd污染显著,超过国家土壤三级标准。微宇宙系统试验表明,Cd的污染状况分析与现场采样分析结果一致;沉降是Cd和Pb在水环境中主要的迁移过程,在试验时长216 h内,Cd大约有61%保留在沉积物中,倾向于停留在水相中,容易通过水体的平流和扩散扩大污染范围,而Pb有99%保留在沉积物中,更容易吸附在悬浮物表面而向沉积物相沉积。研究表明,露天堆放的矿产品的影响是显著的,应尽可能采用集装箱堆放方式,减少矿石直接与雨水接触的机会,封闭污染通道,降低环境效应。Abstract: Based on field research at port mineral yard, leaching of heavy metals as an important port soil, water pollution cannot be ignored. The rules of typical pollutants were simulated by the micro-universe model. In this study, the soil and water samples from 7 heavy metals storage yards of Tianjin Port have been investigated, revealing high Cd pollution in soil. Aquatic microcosm was used to simulate the chemical behavior of lead and cadmium in Tianjin port, and the internal transportation law was found by using the fugacity-based multimedia environmental mathematical model. The results of the model and experiment were consistent, showing that this model is suitable. The results of the model suggest that 61% of the cadmium in the system was sunk into sediment, the rest flowing out with water, and 99% of the lead was sunk into sediment, showing that for cadmium and lead, settling was the main transport process in the aquatic environment, especially for lead. The impact of minerals stored in the open areas is significant, and should be stored in containers without any direct contact with rain. Also, the pollution channel should be kept closed.
-

-
表 1 堆场调研的土壤和水体重金属分析测试结果
Table 1. Analytical results of heavy metal elements in on-site soil and water samples
样品 元素 w/(kg·g-1) 1 2 3 4 5 6 7 标准限值 20 cm表层土壤 Cd 4.2 6.2 - 5.7 64.8 - 56.1 0.84~1.05 Pb 50.3 19.8 - 55.7 69.4 - 20.5 350 60 cm深层土壤 Cd 1.8 3.6 - 2.4 32.0 - 50.0 0.84~1.05 Pb 34.9 ND - 54.7 11.3 - 18.4 350 水体 Cd ND 0.001 0.001 0.002 0.002 ND 0.006 0.1 Ni 0.027 0.023 0.032 0.028 0.001 1.078 0.051 1.0 Hg 0.003 0.018 0.005 0.016 ND 0.038 0.063 0.05 Pb ND 0.004 ND 0.024 ND ND ND 1.0 注:“ND”表示未检出。 表 2 水和沉积物中镉和铅的总浓度值
Table 2. The total concentration of cadmium and lead values of water and sediments
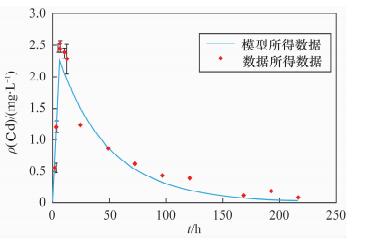
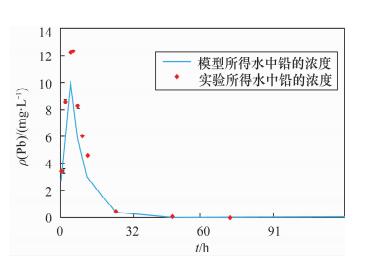
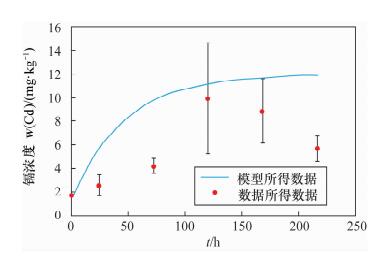
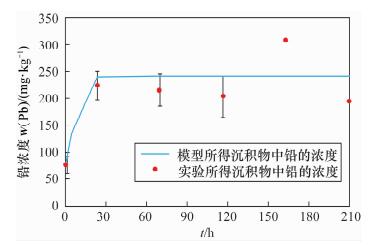
项目时间/h 镉的平均值 铅的平均值 沉积物
(mg/kg)水
(mg/L)沉积物
(mg/kg)水
(mg/L)0 1.70 ND 77.96 ND 1 - 0.54 - 3.40 3 - 1.20 - 8.55 5 - 2.47 - 12.23 6 - 2.52 - 12.36 8 - 2.44 - 8.23 10 - 2.38 - 6.03 12 - 2.29 - 4.62 24 2.55 1.24 2.24×102 0.43 48 - 0.85 - 0.07 72 4.19 0.62 2.16×102 0.01 96 - 0.43 - ND 120 9.93 0.39 2.05×102 ND 144 - ND - ND 168 8.84 0.12 3.09×102 ND 192 - 0.18 - ND 216 5.67 0.06 1.96×102 ND 注:“ND”表示未检出。24~216 h水中铅和镉的浓度由ICP-AES测定,其他时间点样品的浓度由原子吸收分光光度计测定。 表 3 模型中的理化参数
Table 3. Physical and chemical parameters in the model
-
[1] 张培玉.渤海湾近岸海域底栖动物生态学与环境质量评价研究[D].山东:中国海洋大学,2005.
[2] Mackay D, Finizio A, Bidleman T.Octanolair partition coefficient as a predictor of partitioning of semivolatile organic chemicals to aerosols [J].Atmospheric Environ-ment,1997,31(7):2289-2296.
[3] Mackay D, Mirlam D.Application of the QWASI (Quantitative Water Air Sediment Interaction) fugacity model to the dynamics of organic and inorganic chemicals in lakes [J].Chemosphere, 1989, 18: 1343-1365. doi: 10.1016/0045-6535(89)90027-1
[4] 郑建平.海河河口生态需水量研究[J].海河大学学报:自然科学版,2005(3): 518-521. http://www.cnki.com.cn/Article/CJFDTOTAL-HHDX200505007.htm
[5] 王振坤.邻苯二甲酸酯类化合物在海河河口水环境中行为研究[D].天津:天津大学, 2006.
[6] 陈少峰.天津港港池水交换与生态堤岸设计研究[D].天津:天津大学, 2011.
[7] 陈瑜.铅和镉在水生微宇宙中的分布特征[D].长春:吉林大学, 2009.
[8] 吴昊.重金属模型——三峡库区水域典型重金属化学行为的多介质环境模型研究与应用[D].重庆:重庆大学, 2007.
[9] Donald M, Diamond M, Lorna C.A model of the exchange of inorganic chemicals between water and sediments [J].Environmental Science & Technology, 1990, 24: 713-722.
[10] Donald M, Salle Y.A quantitative water, air, sediment interaction (QWASI) fugacity model for describing the fate of chemicals in lakes [J].Chemosphere, 1983, 12: 981-997. doi: 10.1016/0045-6535(83)90251-5
[11] Donald M, Diamond M, Donald P.Development of a mass balance model of the fate of 17 chemicals in the bay of Quinte [J].Journal of Great Lakes Research, 1994, 20: 643-666. doi: 10.1016/S0380-1330(94)71184-9
[12] Diamond M.Development of a fugacity/aquivalence model of mercury dynamics in lakes [J].Water, Air, and Soil Pollution, 1999, 111: 337-357. doi: 10.1023/A:1005062316518
[13] Diamond M, Helen L L. Loadings, dynamics and re-sponse time of seven metals in Hamilton harbor: Results of a mass balance study [J].Water Quality, 1996, 3: 623-641.
[14] Helen L L, Diamond M, Donald M.Application of the QWASI fugacity/aquivalence model to assessing sources and fate of contaminants in Hamilton harbour [J].Journal of Great Lakes Research, 1993, 19: 582-602. doi: 10.1016/S0380-1330(93)71243-5
[15] Donald M. Multimedia Environmental Models: The Fugacity Approach [M].Albany: Lewis Publishers, 2001.
[16] 迟杰,张玄.DDTs在海河干流市区段沉积物/水间迁移行为研究[J].环境科学, 2009,30(8):2376-2380. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ200908037.htm
[17] 王振坤.进出口资源性矿产品港口环境风险模型研究报告[D].天津:天津出入境检验检疫局, 2012.
-



 下载:
下载: