Research of Soil Activated Ions Extractant and Preliminary Test Results
-
摘要:
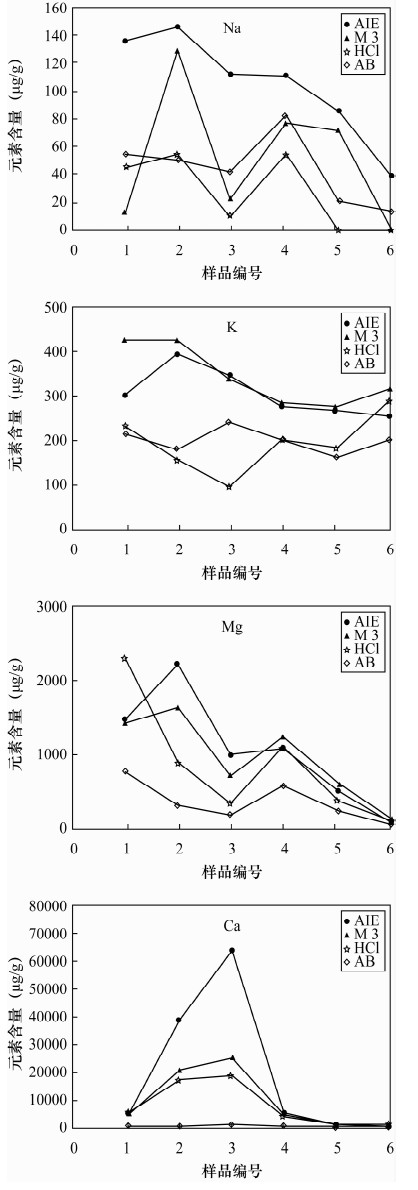
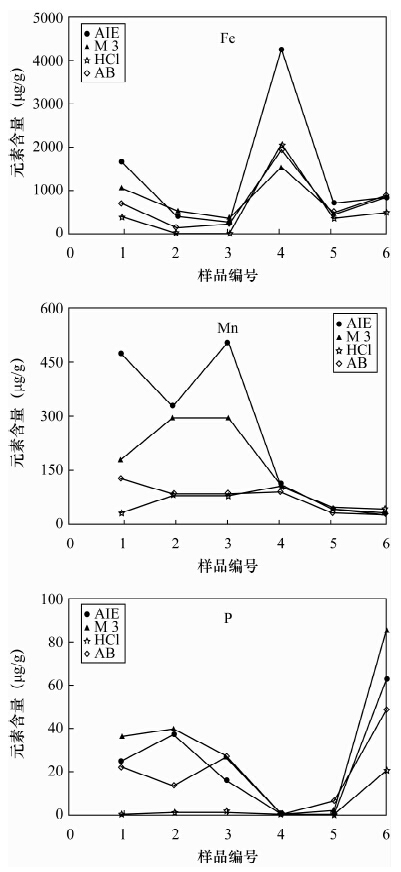
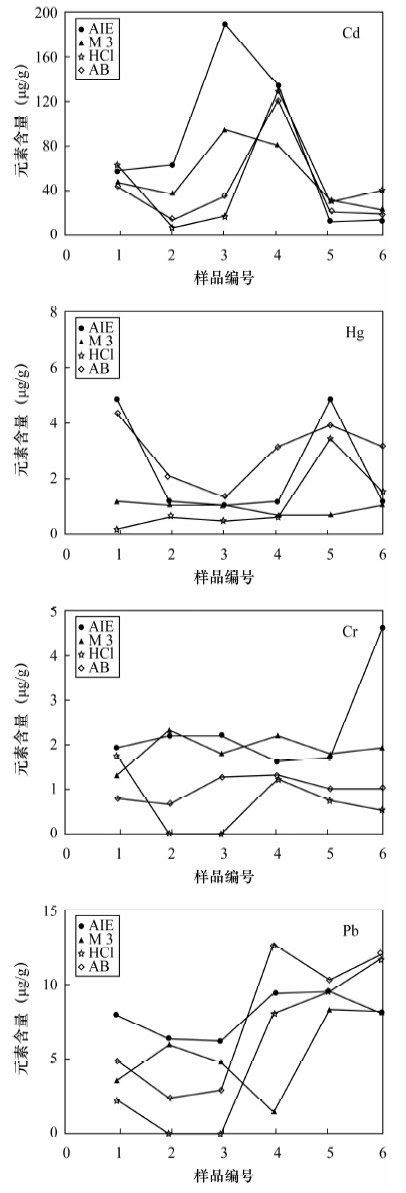
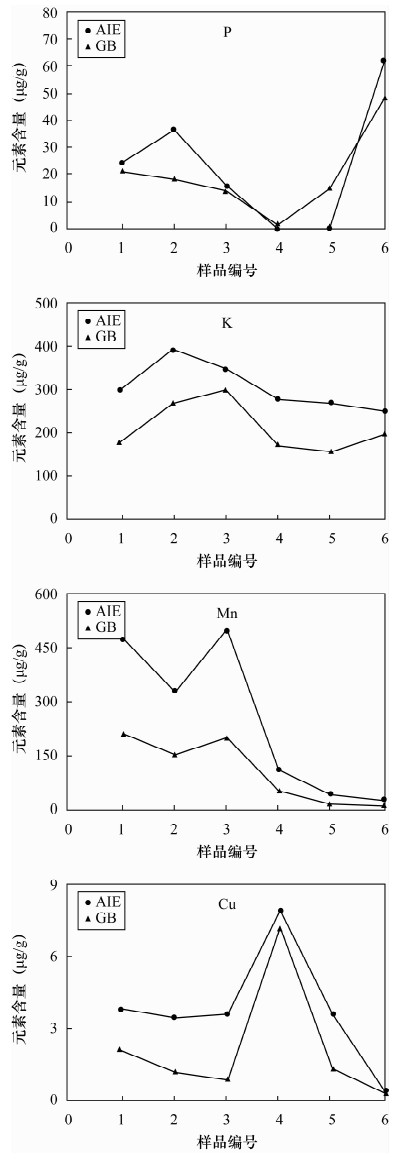
土壤的活性组分能够反映土壤实际污染状况及对环境的危害,选择适当的土壤浸提剂是准确评价土壤活性组分的关键技术,已有的提取剂局限于不同元素和不同土壤类型,提取步骤繁琐,实验周期长,重现性不高。本文研制了一种提取土壤中活性组分的新型提取剂——AIE,提取剂组成为0.25 mol/L醋酸-0.25 mol/L醋酸铵-0.005 mol/L DTPA-0.2%对苯二酚混合溶液。国家标准物质提取实验表明,AIE提取剂能够有效提取土壤中多种元素的活性组分(有效态磷、有效态钾、有效态锰、有效态铜),具有很好的通用性。AIE提取剂与三种通用提取剂(Mehlich 3、AB-DTPA、盐酸)的实验比较表明,AIE对作物营养组分盐基离子(K、Na、Ca、Mg)的提取效果高于AB-DTPA和盐酸,与Mehlich 3的提取量变化规律基本一致;对Fe、Mn、P和重金属元素的提取值70%高于或相当于其他三种提取剂。应用AIE提取土壤的活性组分,适用于提取作物营养组分和重金属元素,既可提取有效态又可提取缓效态,且样品无需针对不同元素做分别处理,多种元素提取方法一致,比已有的提取剂实验周期缩短3~5倍,有利于大批量样品的分析测试;AIE实际应用的重现性较好,大多数元素提取量的相对标准偏差低于8%;AIE的缓冲能力强,提取液的pH值升高幅度(0.07~0.9)均未超过缓冲溶液的缓冲范围,可同时适用于酸性和碱性土壤。总体上AIE通用性优于Mehlich 3和AB-DTPA提取剂,是已有提取分析方法的补充和完善。
Abstract:Active constituents in soil can reflect the status of the actual pollution and harm to the environment, and selection of the appropriate extractant is a key technology to accurately evaluate active soil components. The existing extractant method has been limited to different elements and different soil types with complicated extraction steps, a long experimental period and poor reproducibility. A new type of activated ions extractant (AIE) has been developed in this study. The new extracting solution, already designated AIE, is composed of 0.25 mol/L CH3COOH-0.25 mol/L NH4OAc-0.005 mol/L DTPA-0.2% C6H4(OH)2. Extraction experiment results of the national standard substances show that AIE extraction agent can effectively extract active components (available P, available K, available Mn and available Cu) in soil with good versatility. AIE extracting results in comparison with three extraction extractants such as Mehlich 3, AB-DTPA and HCl, show that the extraction effect of AIE on nutrition component base cations (K, Na, Ca, Mg) is higher than that of AB-DTPA and HCl extraction agents, and the extraction effect of AIE and Mehlich 3 have the same trend. Extraction effects of AIE on Fe, Mn, P and heavy metals were 70% better than the three kinds of extracting agents mentioned. AIE was suitable for the extraction of nutrition components and heavy metals, which can extract the available state and slow release state. The extraction method of different elements was the same and no need to process separately, and the experimental period was 3-5 times less than the existing extractant method, which was favourable for the analysis of quantities of samples. AIE extraction results have good reproducibility, and relative standard deviations of the other elements except Na were lower than 8%. AIE has strong buffering capacity, and a pH value range of 0.07-0.9 is in the buffering capacity of buffer solution, which is good for acidic and alkaline soils. The versatility is better than that of Mehlich 3 and AB-DTPA. The new extracting agent is a supplement and improvement of existing methods.
-
Key words:
- soil /
- activated ions extractant (AIE) /
- Mehlich 3 /
- AB-DTPA /
- HCl /
- extraction effect
-

-
表 1 土壤样品特性
Table 1. The physical and chemical characteristics of soil samples
样品
编号国家标准
物质编号采样地点 土壤
类型pH 原样简述 1 GBW 07412 辽宁开源 棕壤 5.98 棕色粉砂质壤土,
母岩为花岗岩2 GBW 07413 河南安阳 潮土 8.24 石灰性浅褐色粉砂壤土,
母质为洪、冲积物3 GBW 07414 四川简阳 紫色土 8.14 紫褐色黏性壤土,
母质为砂页岩4 GBW 07415 湖北黄梅 水稻土 5.55 灰色粉砂质黏性壤土,
母质为湖积物5 GBW 07416 江西鹰潭 红壤 5.44 红色粉砂质黏性壤土,
母质为第三系沉积物6 GBW 07417 广州花县 赤红壤 5.44 褐黄色含砂黏性壤土,
母岩为花岗岩表 1 土壤、提取剂及提取液的pH值
Table 1. pH value of soils, extractants and extracted solutions
样品
编号土壤的
pH值AIE提取液
的pH值
(AIE提取剂
pH=4.44)Mehlich 3提取液
的pH值
(Mehlich 3提取剂
pH=2.51)AB-DTPA提取液
的pH值
(AB-DTPA提取剂
pH=7.85)1 5.98 4.51 3.25 8.19 2 8.24 4.91 4.08 8.28 3 8.14 5.34 4.17 8.15 4 5.55 4.53 3.28 8.25 5 5.44 4.53 3.31 8.28 6 5.44 4.56 3.37 8.25 表 2 试制提取剂重现性检验结果
Table 2. Reproducibility of element analysis with AIE extractant
样品编号 元素提取量(μg/g) Hg提取量
(ng/g)Cd Cr Cu Pb Mn Ca K Mg Na 1 207 1.4 10.7 20 364 4042 173 484 88 5.8 2 213 1.5 11.2 22 336 3815 160 447 51 5.5 3 216 1.1 12.3 25 354 3999 178 475 62 5.8 4 209 1.3 11.4 23 353 3972 176 452 67 5.6 5 211 1.4 12.2 24 345 4001 168 465 72 5.6 6 214 1.3 11.8 22 348 3898 172 473 68 5.8 RSD(%) 1.3 7.5 4.3 5.9 2.0 1.7 2.8 2.4 11.7 2.1 -
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] doi: 10.1021/ac50043a017
[16] [17] [18] [19] [20] [21] [22] -



 下载:
下载: