Sample Pre-treatment Technologies for Gas Composition Analysis of Natural Gas Hydrates
-
摘要: 天然气水合物仅在相对低的温度和高压条件下稳定存在,一旦脱离其稳定条件就将分解成气体和水而不复存在,因此水合物样品的储存与制备等相关前处理过程对其气体组成的准确测定十分重要。本文实验研究了天然气水合物在常压条件下的最佳储存温度、最佳分解方法、分解气的最佳收集与储存方式,以及非水合物气体的排除等样品前处理技术。结果表明:天然气水合物在常压下低于-100℃储存为妥;样品在进行分解脱气时,“顶空法”和“注射器法”适用性较广,“排水法”不适用于含CO2的水合物样品,且样品分解前最好于-80℃放置片刻以去除表面吸附的非水合物气体。水合物分解气体的储存应尽量避免使用铝塑气袋,建议采用丁基橡胶塞密封的玻璃顶空瓶,并于5天内完成气体组成测定为佳。Abstract: Natural gas hydrate can only steadily exist at lower temperature and higher pressure, otherwise it will decompose into gas and water. Hereby, the pre-treatments (sample preservation, preparation, etc.) are very important to the accurate measurement of gas components of gas hydrate. Described in this paper are the pre-treatment technologies of gas hydrate that were studied experimentally, mainly including the optimal preservation temperature under atmospheric pressure, the optimal decomposition methods, the optimal ways of gas collecting and storing, and the removal methods of non-hydrate gases. The results indicate that the best temperature for gas hydrate storage is less than -100℃ under atmospheric pressure. The headspace method and syringe method can be widely used in hydrate-bound gases′ decomposition and collection, however, the drainage method was not suitable for hydrate samples containing CO2. It was more beneficial to place the sample at -80℃ to remove the non-hydrate gases absorbed on the surface of the specimen. In addition, the use of a glass bottle with butyl rubber plug for storage of hydrate decomposition gases instead of aluminum-plastic air bag is preferential, and the optimum time to finish the analysis of molecular compositions is within 5 days.
-

-
表 1 松散粉末状水合物在不同温度下的分解情况
Table 1. The decomposition of loose powdered gas hydrate at different temperature
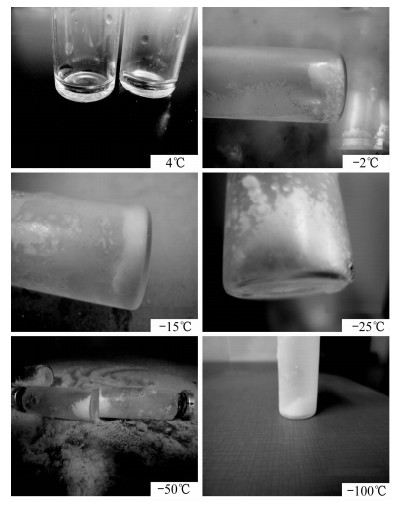
储存时间 储存温度 4℃ -2℃ -15℃ -25℃ -50℃ -100℃ 3 h 液体 半湿颗粒 较干粉末 较干粉末 松散干粉末 松散干粉末 1天 液体 大块冰团 略湿粉末 略湿粉末 松散干粉末 松散干粉末 5天 液体 大块冰团 大块冰颗粒 略湿小颗粒 松散干粉末 松散干粉末 10天 液体 大块冰团 大块冰颗粒 略湿小颗粒 松散干粉末 松散干粉末 瓶内气压 < 0.1 MPa < 0.1 MPa < 0.1 MPa 约0.1 MPa > 0.1 MPa > 0.1 MPa 分解情况 完全分解 大量分解 少量分解 较少分解 未见明显分解 未见明显分解 表 2 块状水合物在不同温度下的分解情况
Table 2. he decomposition of massive gas hydrate at different temperature
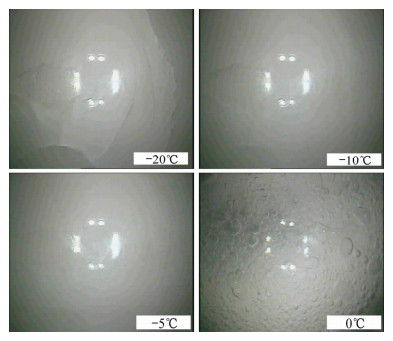
储存时间 储存温度 -20℃ -10℃ -5℃ 0℃ 3天 块状,
表面有裂纹表面略微熔解,
裂纹逐渐消失- 表面有大量
气泡、液滴6天 - - 表面略微熔解,
裂纹基本消失- 釜内压力 -0.08 MPa -0.08 MPa -0.06 MPa 0.58 MPa 分解情况 未见明显分解 未见明显分解 未见明显分解 显著分解 表 3 不同分解方式所得水合物的气体组成
Table 3. Molecular compositions of hydrates-bound gases obtained from different decomposition methods
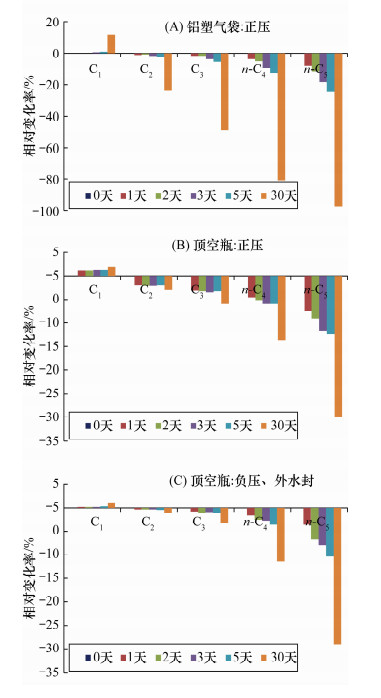
分析项目 不同样品分解方法测定的气体组成 wB/% 排水法 真空顶空法 注射器法 C1 69.97 68.78 68.44 C2 11.89 11.55 11.85 C3 16.49 14.97 14.74 n-C4 0.95 0.90 0.92 n-C5 0.05 0.05 0.07 CO2 0.64 3.74 3.98 -
[1] Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd Edition. Boca Raton: CRC Press, 2007.
[2] 赵祖斌,杨木壮,沙志彬.天然气水合物气体成因及其来源[J].海洋地质动态, 2001, 17(7): 38-41. http://www.cnki.com.cn/Article/CJFDTOTAL-HYDT200107008.htm
[3] 孟庆国.海洋天然气水合物模拟实验研究[D].青岛:青岛大学, 2009.
[4] 张凌,宁伏龙,蒋国盛,吴翔,窦斌,涂运中.海洋水合物钻探取心与处理现状分析[J].探矿工程(岩土钻掘工程), 2009(Z1): 100-103. doi: 10.3969/j.issn.1672-7428.2009.z1.023
[5] 张凌,蒋国盛,宁伏龙,吴翔,窦斌,涂运中.国外天然气水合物岩心处理分析技术综述[J].地质科技情报, 2009,28(1): 123-126. http://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ200901024.htm
[6] Gudmundsson J S, Parlaktuna M. Storage of Natural Gas Hydrate at Refrigerated Conditons [C]//Proceedings of AICHE Spring National Meeting,1992: 27-32.
[7] Milkov A V. Molecular and stable isotope compositions of natural gas hydrates: A revised global dataset and basic interpretations in the context of geological settings [J]. Organic Geochemistry, 2005,36(5): 681-702. doi: 10.1016/j.orggeochem.2005.01.010
[8] Stern L A, Lorenson T D, Pinkston J C. Gas hydrate characterization and grain-scale imaging of recovered cores from the Mount Elbert gas hydrate stratigraphic test well, Alaska North Slope [J]. Marine and Petroleum Geology, 2009, doi:10.1016/j.marpetgeo.
[9] Lorenson T D, Collett T S, Hunter R B.Gas geochemistry of the Mount Elbert gas hydrate stratigraphic test well, Alaska North Slope[J]. Marine and Petroleum Geology, 2010. doi:10.1016/j.marpetgeo.2010.02.007 .
[10] Vaular E N, Barth T, Haflidason H. The geochemical characteristics of the hydrate-bound gases from the Nyegga pockmark field, Norwegian Sea[J]. Organic Geochemistry, 2010,41(5): 437-444.
[11] Charlou J L, Fouquet Y, Donval J P, Auzende J M, Baptiste P J, Stievenard M, Michel S. Mineral and gas chemistry of hydrothermal fluids on an ultrafast spreading ridge: East Pacific Rise, 17° to 19° (Naudur cruise, 1993)—phase separation processes controlled by volcanic and tectonic activity [J].Journal of Geophysical Research, 1996, 101: 15899-15919. doi: 10.1029/96JB00880
[12] 贺行良,刘昌岭,孟庆国,祝有海,业渝光,夏宁.祁连山冻土区天然气水合物气体组分的气相色谱法测定[J].地质通报,2011, 30(12): 7-12. http://www.cnki.com.cn/Article/CJFDTOTAL-ZQYD201112008.htm
[13] 卢振权,祝有海,张永勤,文怀军,李永红,贾志耀,王平康,李清海.青海祁连山冻土区天然气水合物的气体成因研究[J].现代地质,2010,24(3): 581-588. http://www.cnki.com.cn/Article/CJFDTOTAL-XDDZ201003024.htm
[14] Pape T, Bahr A, Rethemeyer J, Kessler J D, Sahling H, Hinrichs K U, Klapp S A, Reeburgh W S, Bohrmann G. Molecular and isotopic partitioning of low-molecular-weight hydrocarbons during migration and gas hydrate precipitation in deposits of a high-flux seepage site [J]. Chemical Geology, 2010,269(3-4): 350-363. doi: 10.1016/j.chemgeo.2009.10.009
[15] Milkov A V, Claypool G E, Lee Y J, Bohrmann G, Borowski W S, Tomaru H. Gas hydrate systems at Hydrate Ridge Offshore Oregon inferred from molecular and isotopic properties of hydrate-bound and void gases[J].Geochimica et Cosmochimica Acta,2005,69(4): 1007-1026. doi: 10.1016/j.gca.2004.08.021
[16] 业渝光,刘昌岭,贺行良,孟庆国,孙始财.水合物相平衡原位监测实验装置[P].中国:ZL 2011-2-0099338.9 [2011-01-07].
[17] 孟庆国,刘昌岭,业渝光,陈强.不同体系中甲烷水合物储气特性实验研究[J].世界科技研究与发展,2011, 33(1): 25-28. http://www.cnki.com.cn/Article/CJFDTOTAL-SJKF201101008.htm
[18] 贺行良,夏宁,刘昌岭,王江涛,孟庆国.FID/TCD并联气相色谱法测定天然气水合物的气体组成[J].分析测试学报,2012,31(2): 206-210. http://www.cnki.com.cn/Article/CJFDTOTAL-TEST201202019.htm
[19] 刘光启,马连湘,刘杰.化学化工物性数据手册(有机卷、无机卷)[M].北京:化学工业出版社,2002.
-



 下载:
下载: