Carbon and Oxygen Isotope Analysis of Trace Carbonate by Kiel Ⅳ-IRMS Using On-line Dual Technique
-
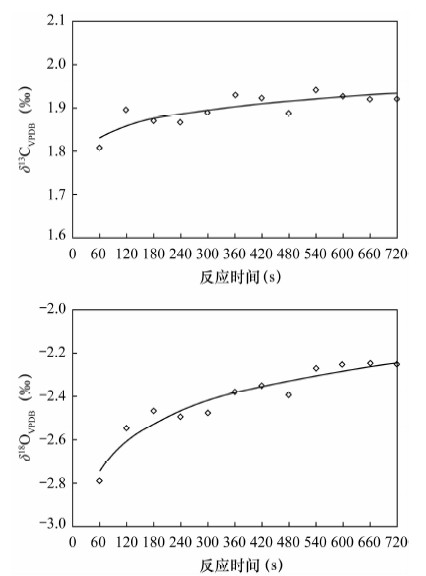
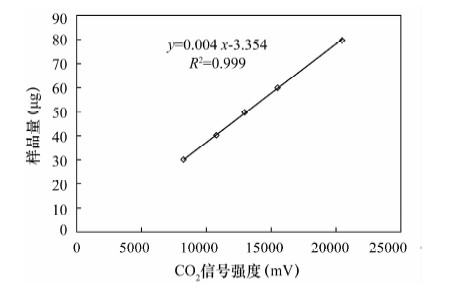
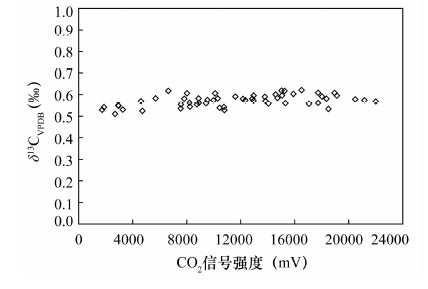
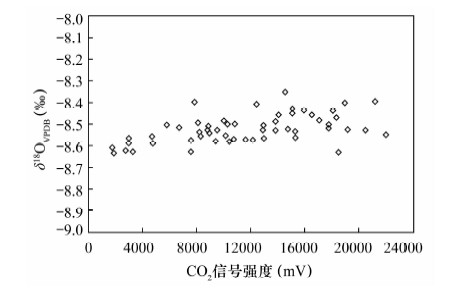
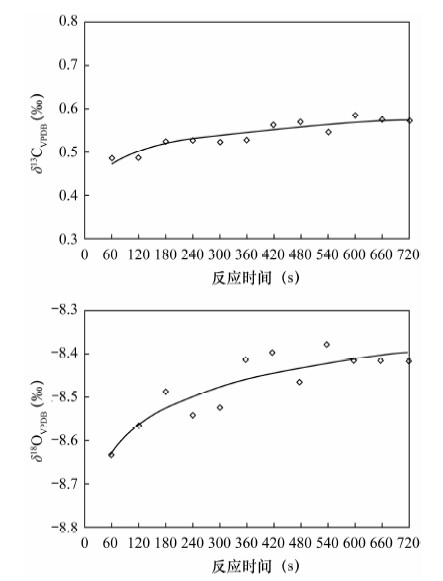
摘要: 石笋能重建百年、十年的气候事件,为达到空间高分辨率,对微量碳酸盐的检测提出了更高要求,传统磷酸酸解法的样品用量大(约10 mg)已经无法满足微量样品的分析,而激光探针质谱分析方法需对检测结果进行校正。本文采用Kiel Ⅳ-IRMS双路在线分析技术对微量碳酸盐样品的碳、氧同位素进行检测研究其可行性,并以GBW 04405和NBS 19为例研究了不同样品量的碳酸盐标准样品在不同反应时间对同位素分馏的影响。结果表明,由于标准样品所需的反应时间不同,从而导致同位素分馏值的差异。对样品量为4~85 μg的标准样品GBW 04405进行检测,δ13C、δ18O测量值分别为0.574‰±0.027‰、-8.519‰±0.065‰,与推荐值0.57‰±0.03‰、-8.49‰±0.14‰基本一致,表明该方法能够满足微量碳酸盐测试的要求。将Kiel Ⅳ-IRMS双路在线分析技术与Gasbench Ⅱ-IRMS检测方法进行对比,对于标准样品GBW 04405,Kiel Ⅳ-IRMS所用样品量约为50 μg,δ13C、δ18O测量值分别为0.576‰±0.012‰、-8.501‰±0.050‰,Gasbench Ⅱ-IRMS所用样品量约为140 μg,δ13C、δ18O测量值分别为0.569‰±0.034‰、-8.590‰±0.099‰。表明Kiel Ⅳ-IRMS方法相比于GasbenchⅡ-IRMS方法所需样品量少,精度高,结果重现性好,该方法在碳酸盐样品的应用上能达到空间高分辨率。
-
关键词:
- 微量碳酸盐 /
- 碳同位素 /
- 氧同位素 /
- Kiel Ⅳ-IRMS双路在线分析 /
- Gasbench Ⅱ-IRMS在线分析
Abstract: Stalagmites can rebuild the paleo-climate of decades and hundred years, which need more accurate detection of trace carbonates in order to achieve high spatial resolution. However, the traditional phosphate acid method with weight about 10 mg has been unable to meet analysis of trace samples, and the laser microprobe mass spectrometry method requires calibration for test results. Carbon and oxygen isotopes of trace carbonate samples have been detected by using Kiel Ⅳ-IRMS with on-line dual technique. Different carbonate standard reference materials of GBW 04405 and NBS 19 were detected under different reaction times and different weights, and the test results were compared with Gasbench Ⅱ-IRMS results. The results indicate that isotopic fractionation values of different carbonate standard reference materials are different, caused by different reaction times. 55 carbonate standard material of GBW 04405 with weight about 4-85 μg have been analyzed and mean values of δ13C and δ18O are 0.574‰±0.027‰ and -8.519‰±0.065‰ which are consistent with certified values of 0.57‰±0.03‰ and -8.49‰±0.14‰, respectively. 14 carbonate standard material of GBW 04405 have been analyzed by Kiel Ⅳ-IRMS and GasbenchⅡ-IRMS with weight respectively about 50 μg and 140 μg. The results of δ13C and δ18O are 0.576‰±0.012‰ and -8.501‰±0.050‰ detected by Kiel Ⅳ-IRMS, 0.569‰±0.034‰ and -8.590‰±0.099‰ detected by Gasbench Ⅱ-IRMS. The test result of the on-line dual technique consumed less sample, had better precision and were more reproducible than that by GasbenchⅡ-IRMS. The method of the on-line dual technique meets the requirements of trace carbonate detection and carbonate sample applications can be used to achieve high spatial resolution. -

-
表 1 国家标准物质δ13C和δ18O分析结果
Table 1. Analytical results of δ13C and δ18O for national standard materials
标准样品 碳氧同位素值 推荐值 (‰) 测量值(‰) 标准偏差 (‰) 第1次 第2次 第3次 第4次 第5次 平均值 GBW 04405 δ13C 0.57±0.03 0.621 0.585 0.573 0.609 0.586 0.595 0.018 δ18O -8.49±0.14 -8.512 -8.396 -8.548 -8.492 -8.501 -8.490 0.050 GBW 04406 δ13C -10.85±0.05 -10.862 -10.885 -10.846 -10.823 -10.824 -10.848 0.026 δ18O -12.40±0.15 -12.265 -12.468 -12.333 -12.307 -12.357 -12.346 0.076 GBW 04416 δ13C 1.61±0.03 1.569 1.632 1.554 1.582 1.587 1.585 0.029 δ18O -11.59±0.11 -11.587 -11.469 -11.616 -11.500 -11.585 -11.551 0.063 表 2 应用Kiel Ⅳ-IRMS与Gasbench Ⅱ-IRMS检测GBW 04405的分析结果
Table 2. Analytical results of δ 13C and δ 18O for GBW 04405 by Kiel Ⅳ-IRMS and Gasbench Ⅱ-IRMS system
测量次数 δ13CVPDB(‰) δ18OVPDB(‰) Kiel Ⅳ-IRMS Gasbench Ⅱ-IRMS Kiel Ⅳ-IRMS Gasbench Ⅱ-IRMS 1 0.581 0.519 -8.543 -8.671 2 0.572 0.532 -8.576 -8.686 3 0.584 0.548 -8.524 -8.685 4 0.560 0.548 -8.482 -8.674 5 0.575 0.552 -8.55 -8.674 6 0.591 0.560 -8.497 -8.383 7 0.569 0.567 -8.577 -8.618 8 0.559 0.569 -8.565 -8.460 9 0.562 0.570 -8.494 -8.640 10 0.586 0.573 -8.567 -8.533 11 0.583 0.575 -8.569 -8.682 12 0.596 0.578 -8.405 -8.650 13 0.580 0.617 -8.525 -8.474 14 0.562 0.653 -8.481 -8.429 平均值 0.576 0.569 -8.501 -8.590 标准偏差 0.012 0.034 0.050 0.099 -
[1] 郑仰帝,蔡进功.南黄海盆地碳酸盐岩碳氧同位素特征及意义[J].石油实验地质,2013,35(3):307-313. doi: 10.11781/sysydz201303307
[2] 汪福顺,万国江,刘丛强,胥思勤.程海沉积物无机碳、氧同位素相关性及其环境意义[J].矿物学报,2002, 22(2):184-188. http://www.cnki.com.cn/Article/CJFDTOTAL-KWXB200202014.htm
[3] 刘春燕,郑和荣,胡宗全,尹伟,李松.碎屑岩中的碳酸盐胶结特征——以鄂尔多斯盆地南部富县地区延长组长6 砂体为例[J].中国科学(地球科学),2012,42(11):1681-1689. http://www.cnki.com.cn/Article/CJFDTOTAL-JDXK201211007.htm
[4] 陈友良,魏佳,叶永钦,宋昊,孙泽轩.若尔盖铀矿田方解石稀土元素与碳氧同位素地球化学特征及其意义[J].地球科学进展,2012,27(10):1061-1067. http://www.cnki.com.cn/Article/CJFDTOTAL-DXJZ201210004.htm
[5] Christoph S, Augusto M.Stalagmite from the Austrian Alps reveals Dansgaard-Oeschger events during isotope stages 3: Implications for the absolute chronology of Greenland ice cores[J].Earth and Planetary Science Letters, 2002, 203: 507-518. doi: 10.1016/S0012-821X(02)00837-3
[6] Holmgren K, Karlen W.Paleoclimatic significance of the stable isotopic composition and petrology of a Late Pleis-tocene stalagmite from Botswana [J].Quaternary Research, 1995, 43:320-328. doi: 10.1006/qres.1995.1038
[7] Wang Y J, Cheng H, Edwards R L, Kong X G, Shao X H, Chen S T, Wu J Y, Jiang X Y, Wang X F, An Z S. Millennial-and orbital-scale changes in the East Asian monsoon over the past 224000 years[J].Nature, 2008,451:1090-1093. doi: 10.1038/nature06692
[8] Zhang P Z, Cheng H, Edwards R L, Chen F H, Wang Y J, Yang X L, Liu J, Tan M, Wang X F, Liu J H, An C L, Dai Z B, Zhou J, Zhang D Z, Jia J H, Jin L Y, Johnson K R.A test of climate, sun and culture relationships from an 1810-year Chinese cave record [J].Science, 2008, 322:940-942. doi: 10.1126/science.1163965
[9] Cai Y J, Tan L C, Cheng H, An Z S, Edwards R L, Kely M J, Kong X G, Wang X F.The variation of summer monsoon precipitation in central China since the last deglaciation[J].Earth and Planetary Science Letters, 2010,291: 21-31. doi: 10.1016/j.epsl.2009.12.039
[10] Cheng H, Edwards R L, Broecker W S, Denton G H, Kong X G, Wang Y J, Zhang R, Wang X F.Ice age terminations[J].Science, 2009,326:248-252. doi: 10.1126/science.1177840
[11] Baker A, Caseidine C J, Gilmour M A, Charman D, Proctor C J, Hawkesworth C J, Phillips N.Stalagmite luminescence and peat humification records of palaeomoisture for the last 2500 years[J].Earth and Planetary Science Letters, 1999, 165: 157-162. doi: 10.1016/S0012-821X(98)00258-1
[12] Fleimanna D, Burnsb S J, Neffc U, Mudelseed M, Manginie A, Mattera A.Palaeoclimatic interpretation of high-resolution oxygen isotope profiles derived from annually laminated speleothems from Southern Oman[J].Quaternary Science Reviews,2004,23:935-945. doi: 10.1016/j.quascirev.2003.06.019
[13] 郑淑蕙,郑斯成,莫志超.稳定同位素地球化学分析[M].北京:北京大学出版社,1986.
[14] 黄俊华,胡超涌,周群峰,杨桂芳.激光探针质谱分析碳酸盐碳、氧同位素技术[J].矿物岩石地球化学通报,2001,20(4):472-474. http://www.cnki.com.cn/Article/CJFDTOTAL-KYDH200104075.htm
[15] 何道清.碳酸盐岩碳、氧同位素分析激光微取样技术[J].西南石油学院学报,2003,25(1):12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-XNSY200301003.htm
[16] 杜广鹏,王旭,张福松.GasBench Ⅱ顶空瓶内空气背景对<100μg碳酸盐中碳氧同位素在线测定的影响及校正方法初探[J].岩矿测试,2010,29(6):631-638. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS201006002.htm
[17] 邓文峰,韦刚健,李献华.不纯碳酸盐碳氧同位素组成的在线分析[J].地球化学, 2005,34(5):495-500. http://www.cnki.com.cn/Article/CJFDTOTAL-DQHX200505007.htm
[18] 曹军骥,王亚强,张小曳,李顺诚,何健辉,曹蕴宁,李杨.大气中碳酸盐的碳同位素分析及其来源指示意义[J].科学通报,2004,49(17):1785-1788. doi: 10.3321/j.issn:0023-074X.2004.17.016
[19] 吴静淑,李金城,朱井泉.连续测定方解石和白云石中碳、氧同位素比值的方法及其意义[J].岩石矿物学杂志,1990,9(2):174-179. http://www.cnki.com.cn/Article/CJFDTOTAL-YSKW199002011.htm
-



 下载:
下载: