Confinement Synthesis of 1T Molybdenum Disulfide at the Interlayer of Montmorillonite and the Adsorption Properties of Heavy Metal Ions
-
摘要:
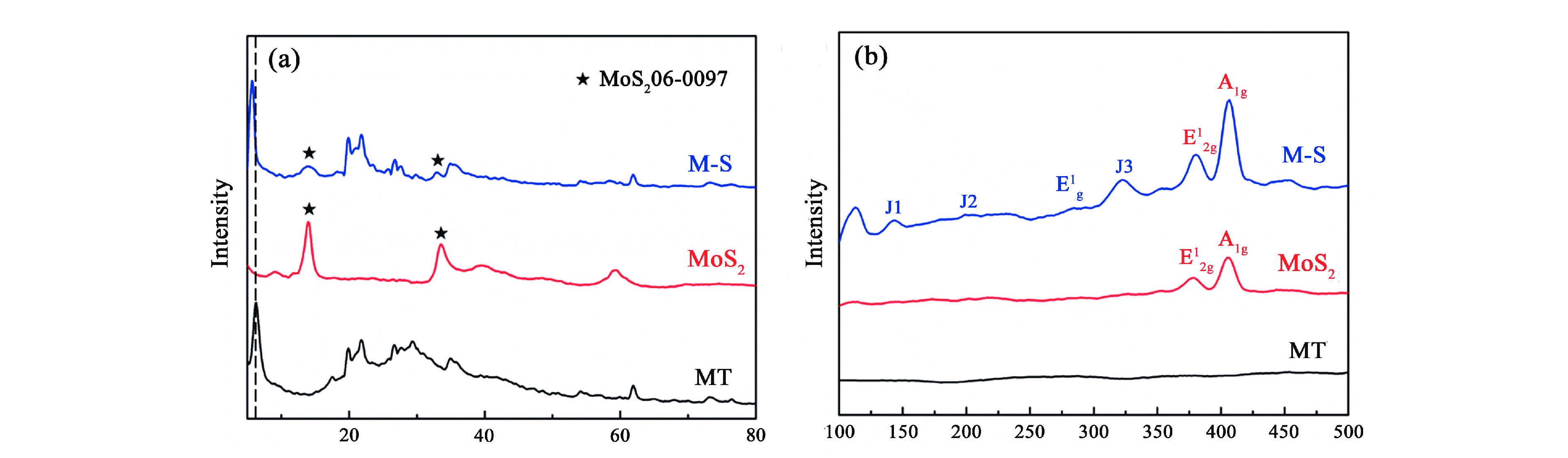
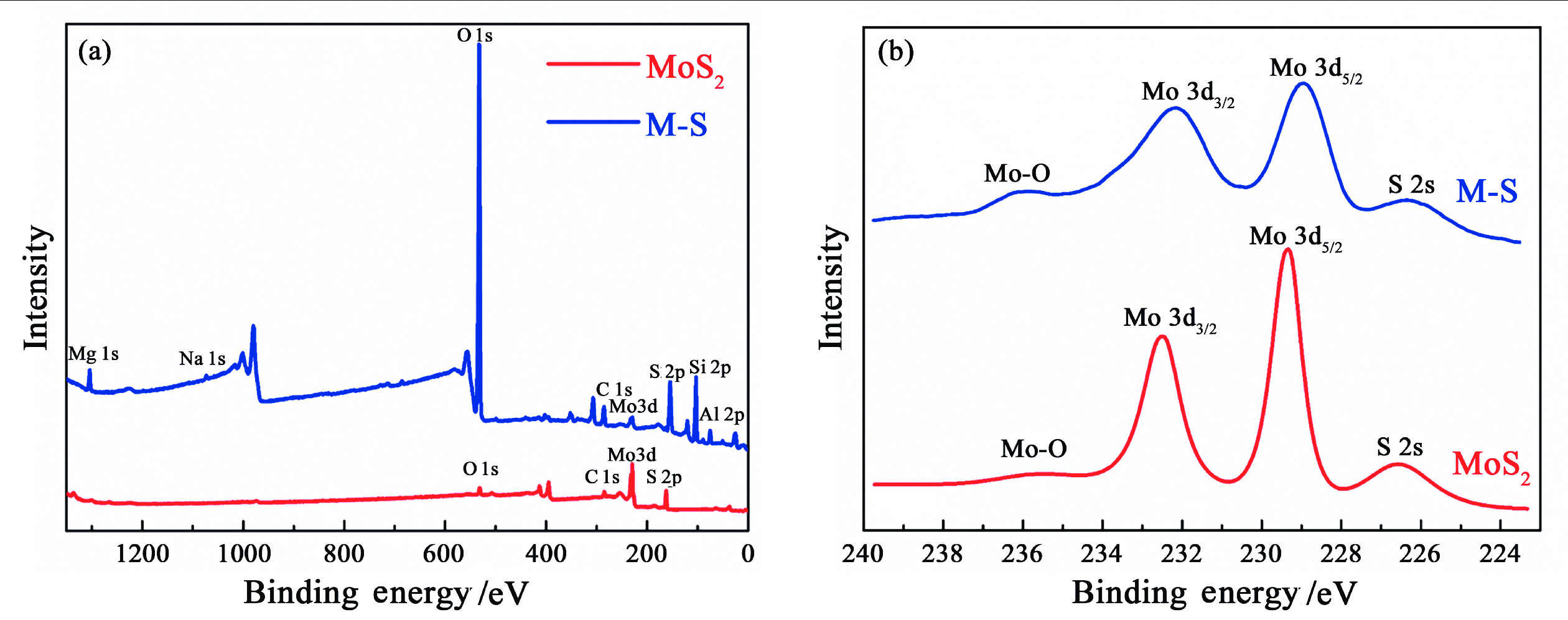
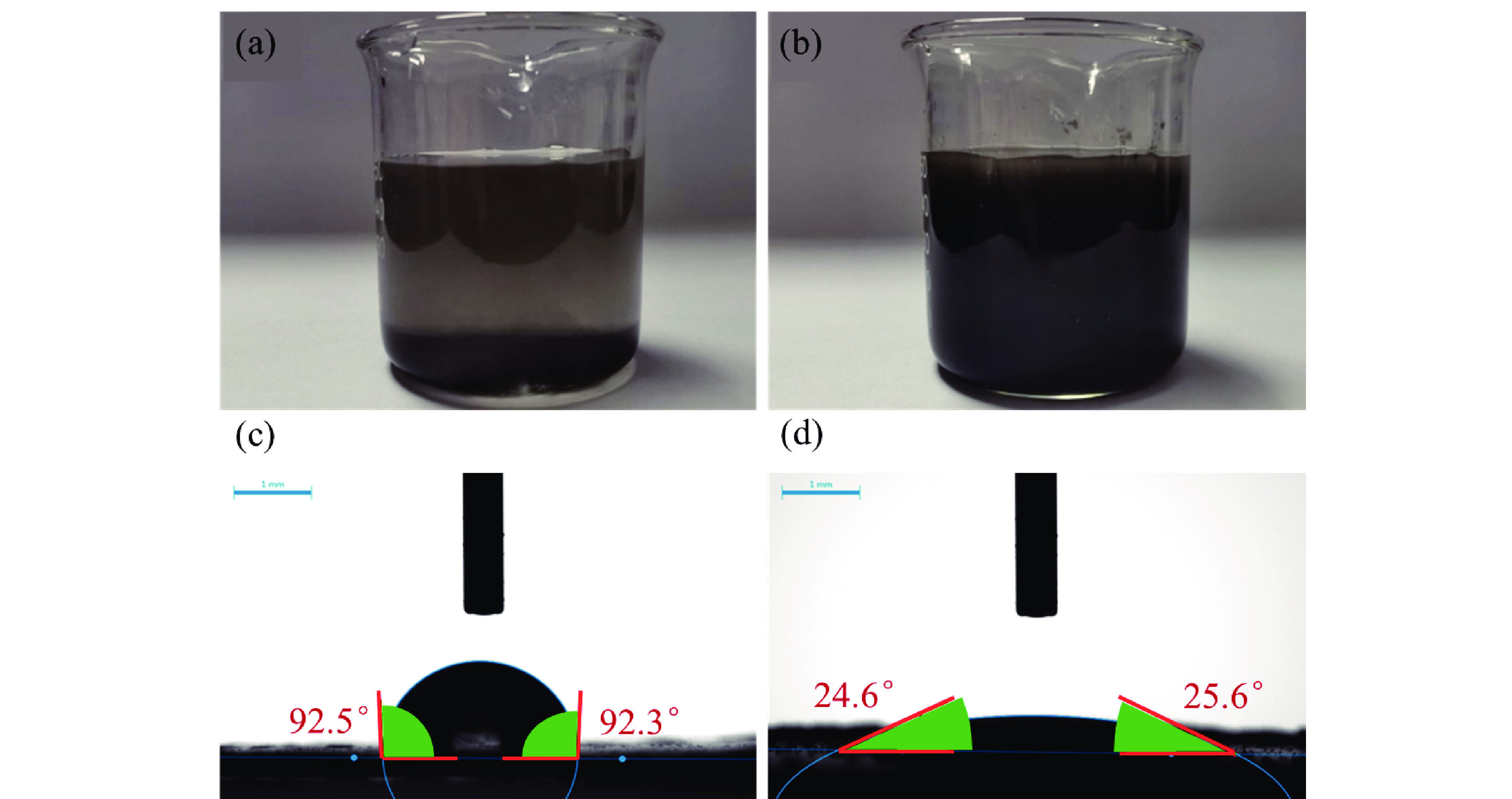
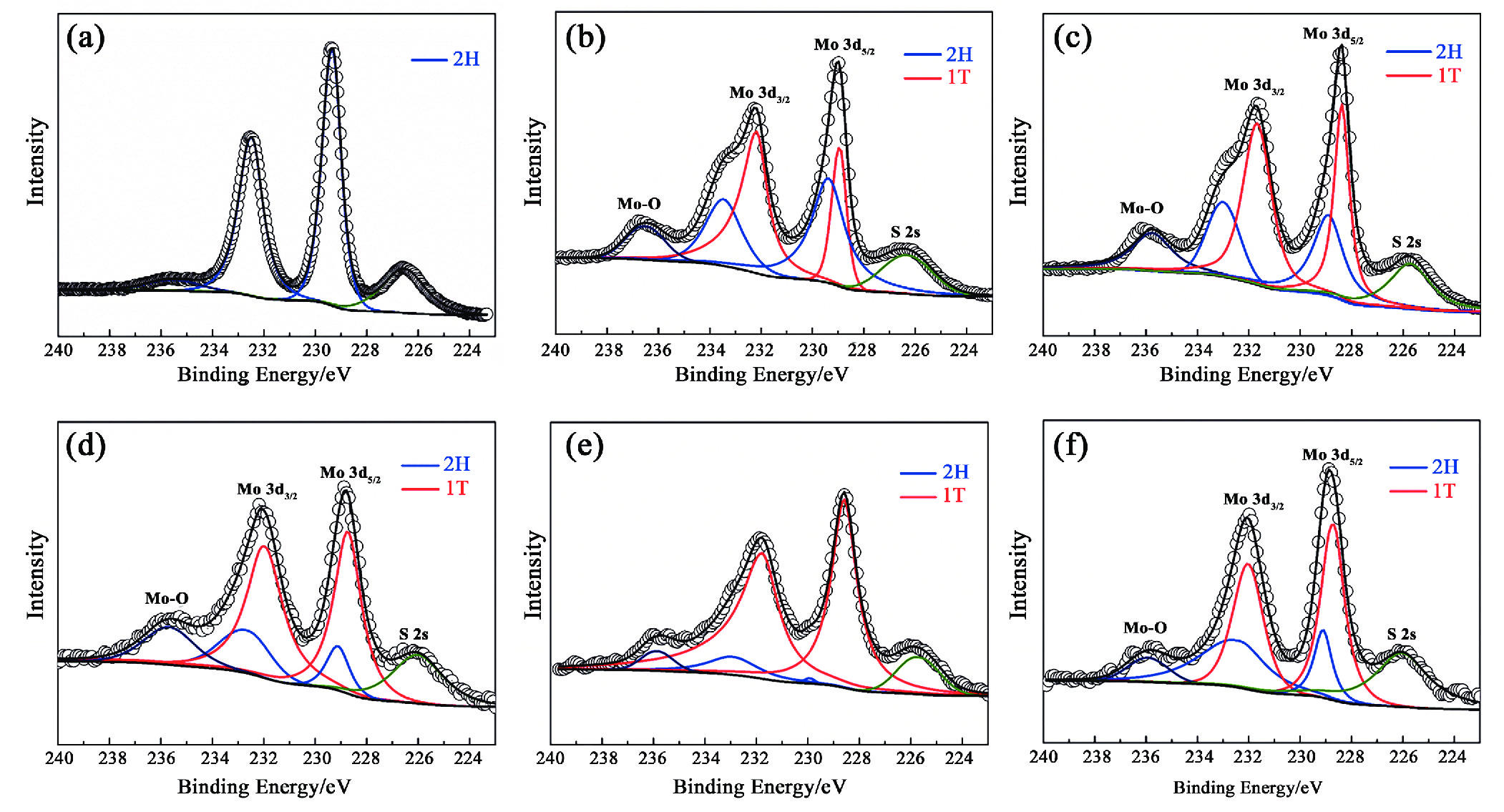
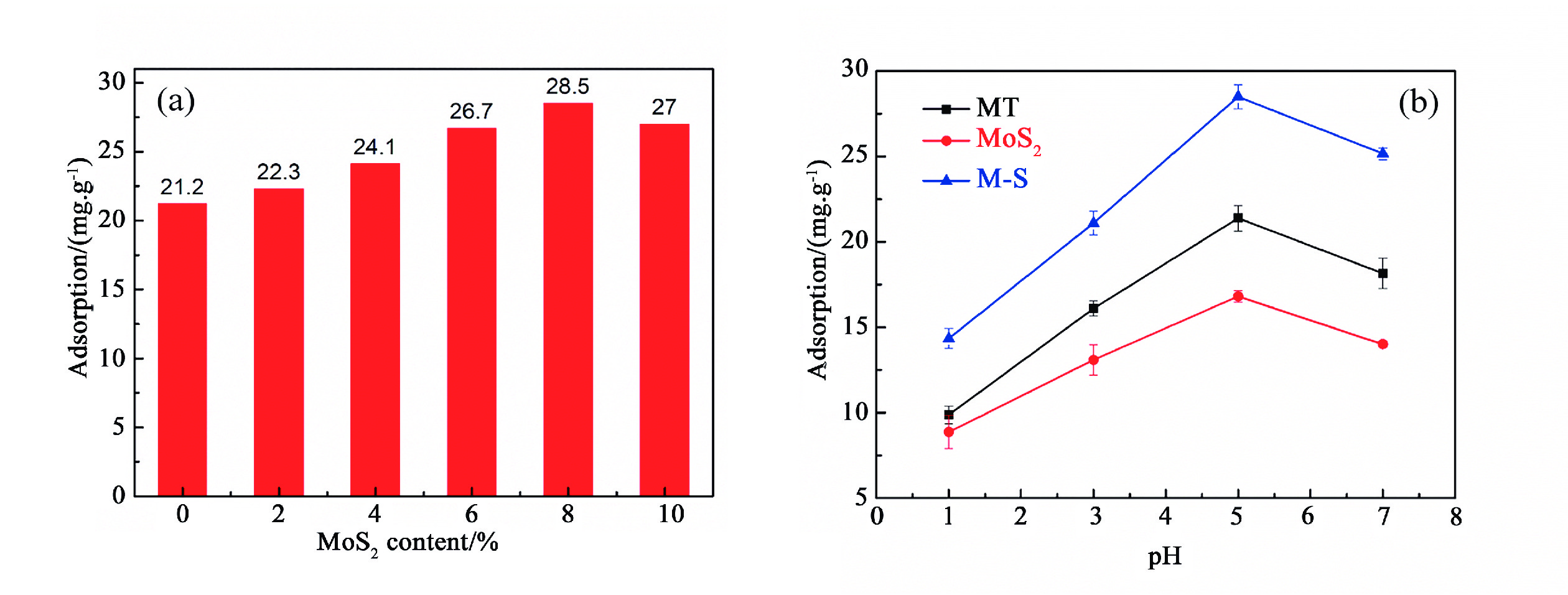
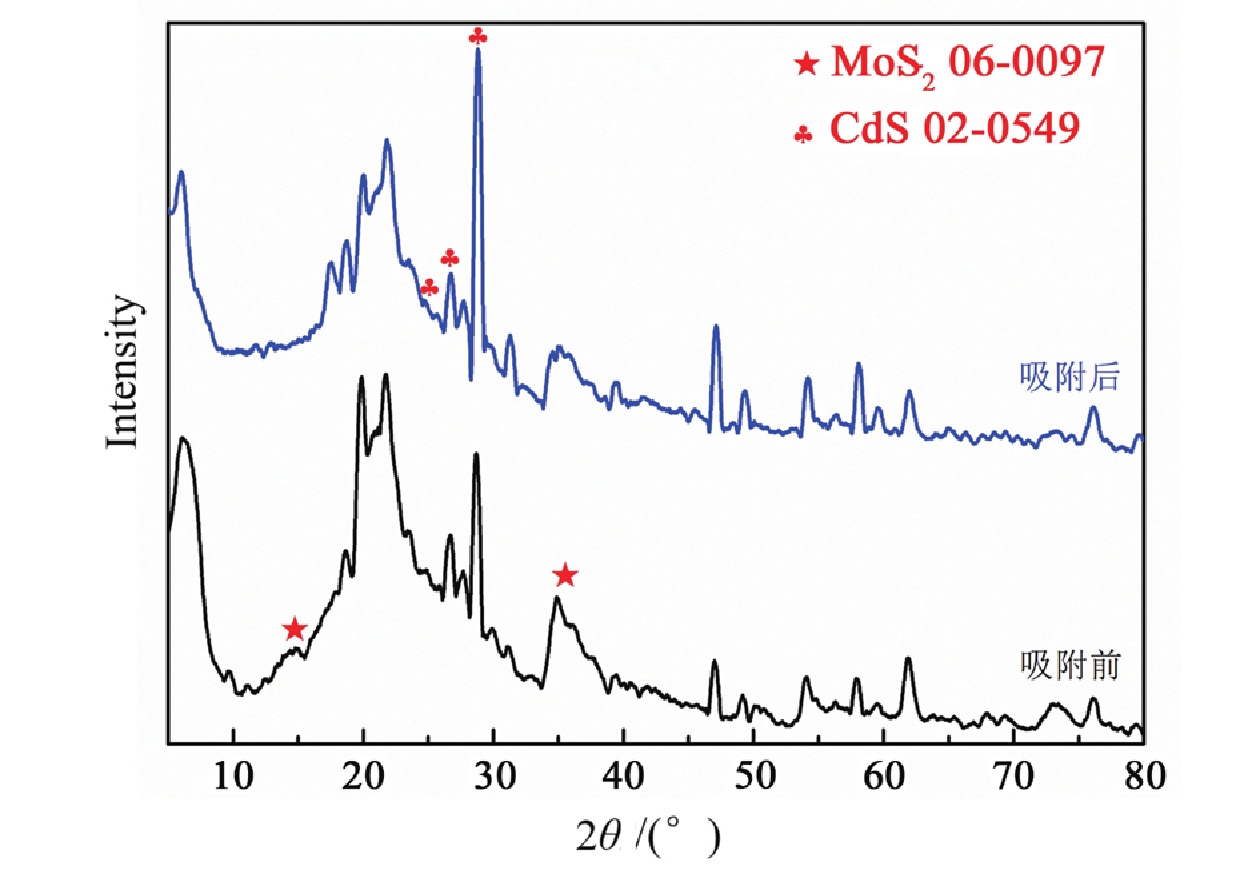
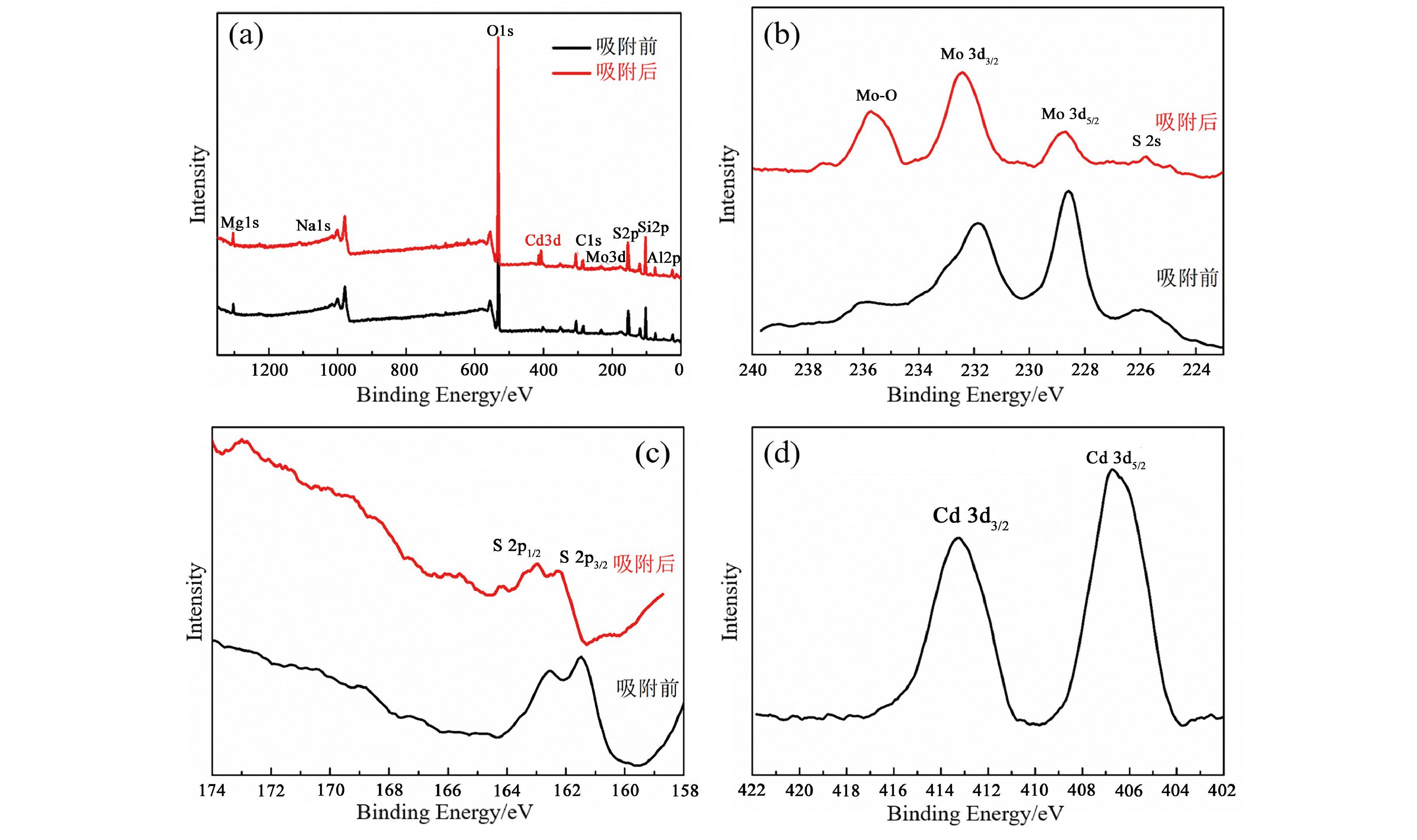
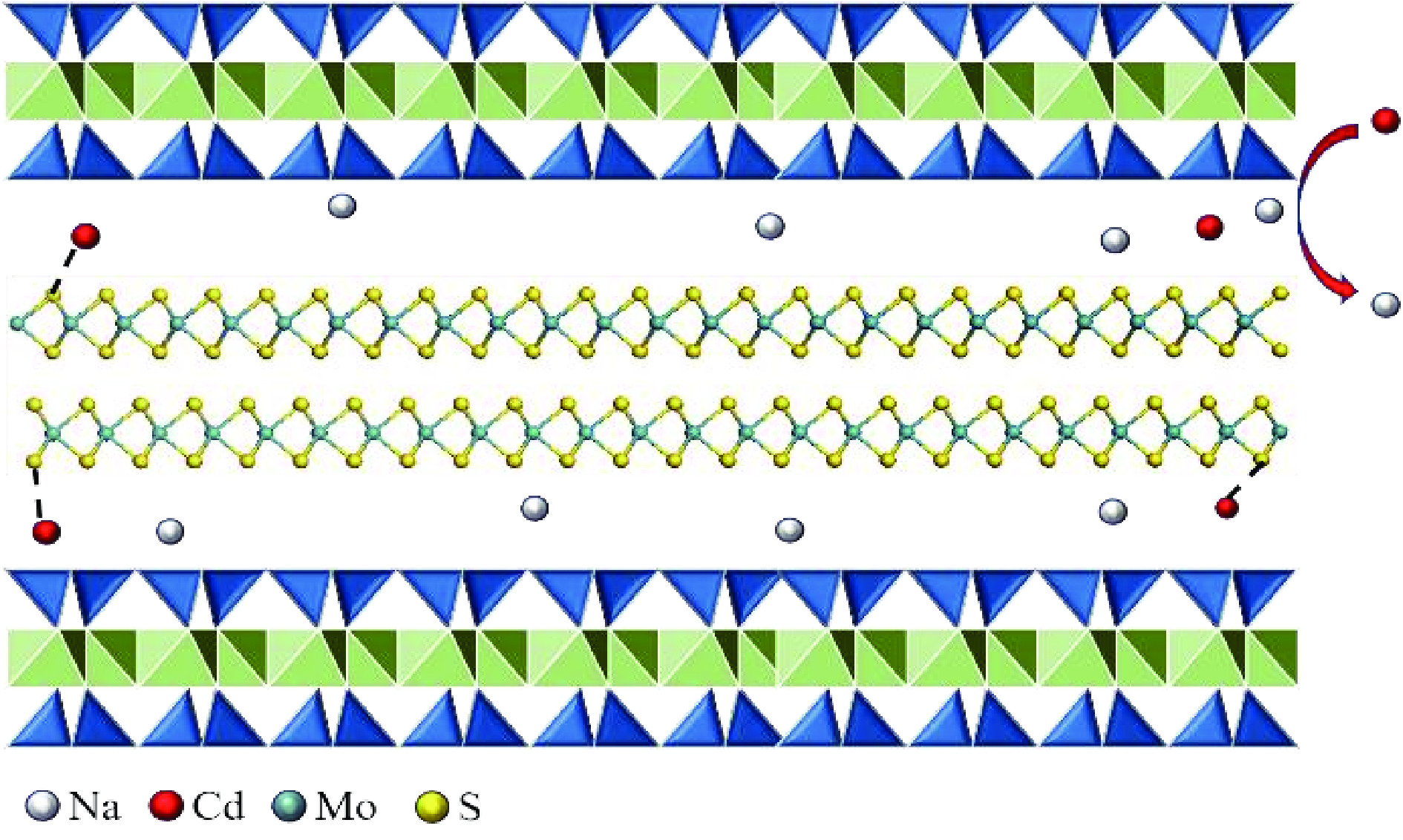
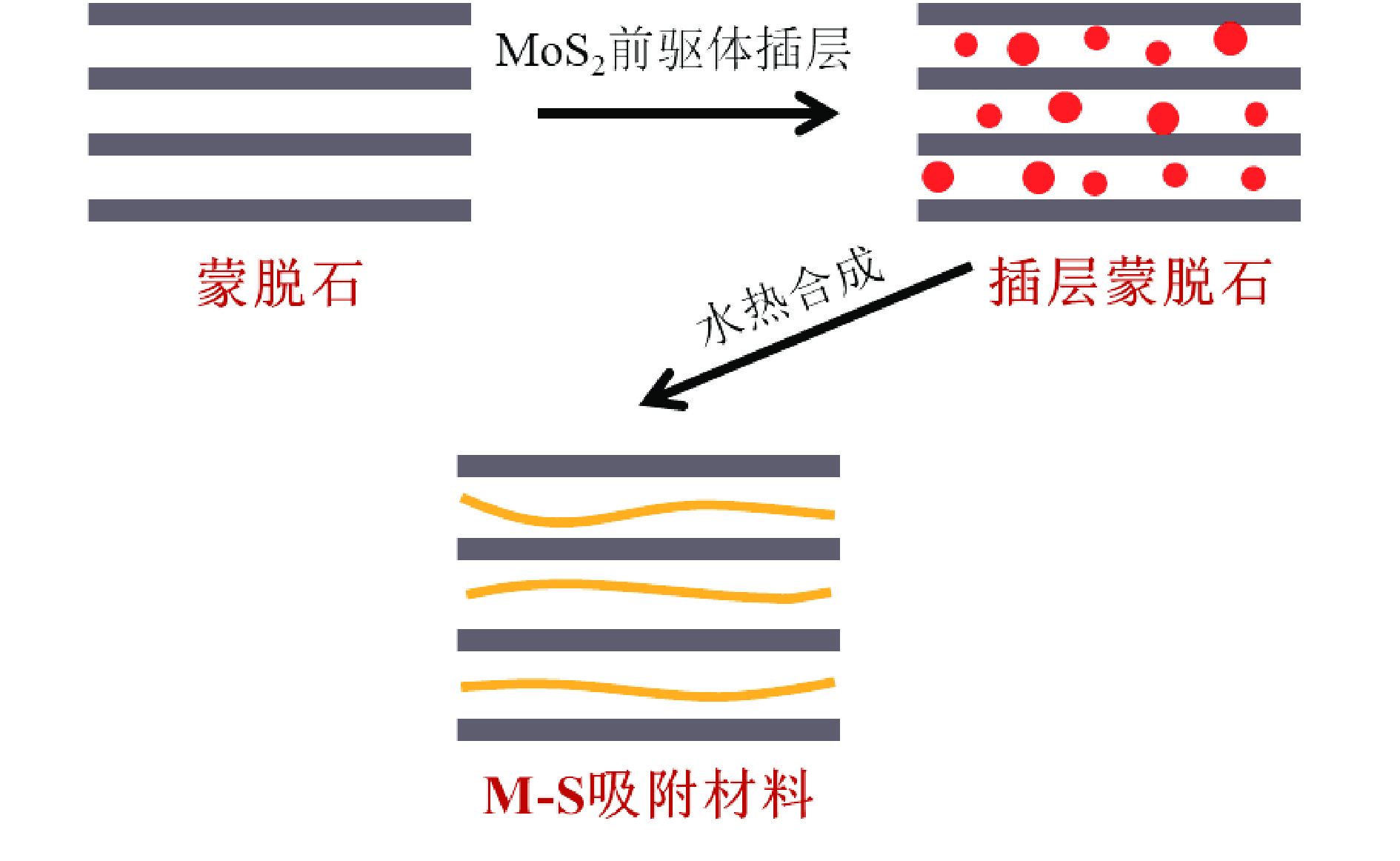
1T型二硫化钼(MoS2)在污水重金属吸附领域有着巨大的潜力,但受限于合成难度大和稳定性差的问题,导致其研究和应用难以取得突破进展。在丰富、廉价的天然层状矿物蒙脱石的纳米层间域中,利用纳米限域效应,实现了1T型MoS2在层间的直接合成,制备了蒙脱石−1T型MoS2层间复合材料(M−S)。通过扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线衍射仪(XRD)、拉曼光谱(Raman)、X射线光电子能谱(XPS)对吸附材料的微观形貌和晶体结构进行了表征,表明产物为蒙脱石−1T型MoS2的复合材料,且1T相占MoS2总量达92.5%。将所制备吸附材料用于水中Cd2+的吸附,考察了溶液pH 值、吸附时间和Cd2+初始浓度对吸附效果的影响。结果表明在pH 值为5.0、吸附时间为5 min、初始质量浓度为250 mg/L时,对Cd2+吸附性能最佳。结合理论模拟得到最佳吸附量为43.9 mg/g,且遵循准二阶动力学方程和Langmuir等温吸附模型。该研究为合成1T型二硫化钼提供了新思路,也为设计高效重金属吸附材料提供了参考。
Abstract:1T molybdenum disulfide (MoS2) has great potential for heavy metal adsorption in sewage. However, due to the difficulty of synthesis and poor stability, the research and application of 1T MoS2 adsorption materials are difficult to achieve breakthroughs. In this paper, the direct synthesis of 1T MoS2 in the interlayer of the rich and cheap natural layered mineral montmorillonite is realized by nano confinement effect, and the adsorbent of montmorillite−1T MoS2 interlayer composite material (M−S) is constructed. By means of scanning electron microscopy (SEM), transmission electron microscopy (TEM), X−ray diffractometer (XRD), Raman spectroscopy, X−ray photoelectron spectroscopy (XPS), the successful synthesis of 1T MoS2 in M−S has been proved, and the content of 1T phase is up to 92.5%. The prepared adsorbent materials were utilized for the efficient removal of Cd2+ from aqueous solutions, and the impacts of pH value, adsorption time, and initial Cd2+ concentration on the adsorption process were systematically investigated. The findings revealed that optimal adsorption performance was achieved at a pH value of 5.0, an adsorption time of 5 minutes, and an initial Cd2+ concentration of 250 mg/L. In conjunction with theoretical simulation, the optimal adsorption capacity was determined to be 43.9 mg/g, in accordance with the quasi−second−order kinetic equation and Langmuir isothermal adsorption model. This study presents a novel approach for synthesizing 1T phase molybdenum disulfide, and also offers valuable insights for the development of efficient heavy metal adsorbents.
-
Key words:
- molybdenum disulfide /
- montmorillonite /
- confinement effect /
- crystal structure /
- heavy metal /
- adsorption
-

-
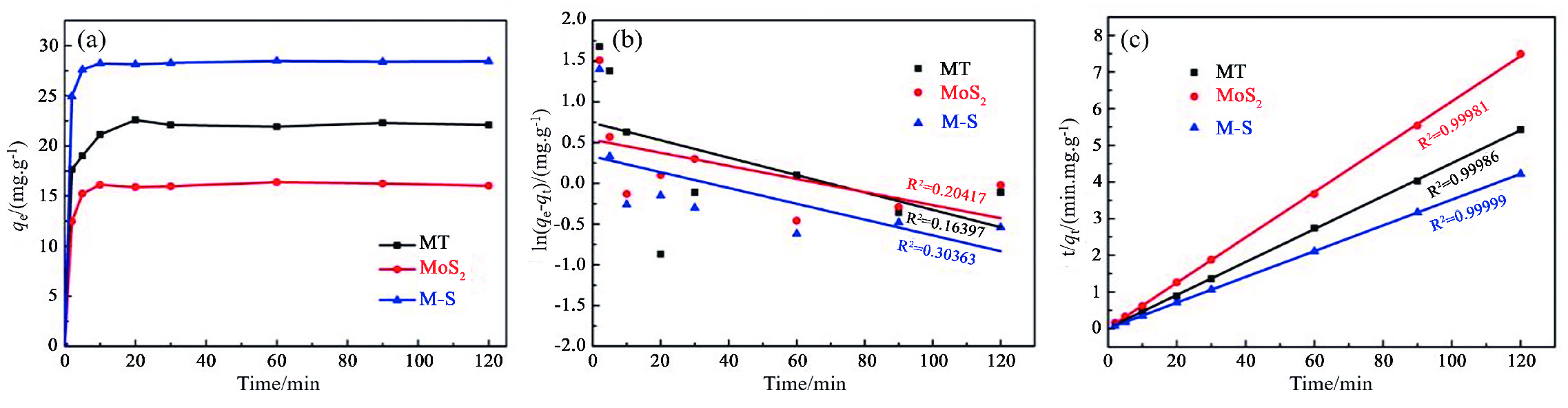
表 1 不同吸附剂对Cd2+的吸附动力学参数
Table 1. Adsorption kinetic parameters of different adsorbents to Cd2+
材料 C0/(mg·L−1) qexp(mg/g−1) 准一阶动力学吸附 准二阶动力学吸附 qcal/(mg·g−1) K1/min R2 qcal/(mg·g−1) K2/(g·mg−1·min−1) R2 MT 50 23 2.1 0.01067 0.16397 20.2 0.1282 0.99862 MoS2 50 17 1.7 0.00803 0.20417 16.2 0.3019 0.98072 M−S 50 29 1.4 0.00969 0.30363 28.5 0.2441 0.99988 表 2 各吸附剂对Cd2+的吸附等温线参数
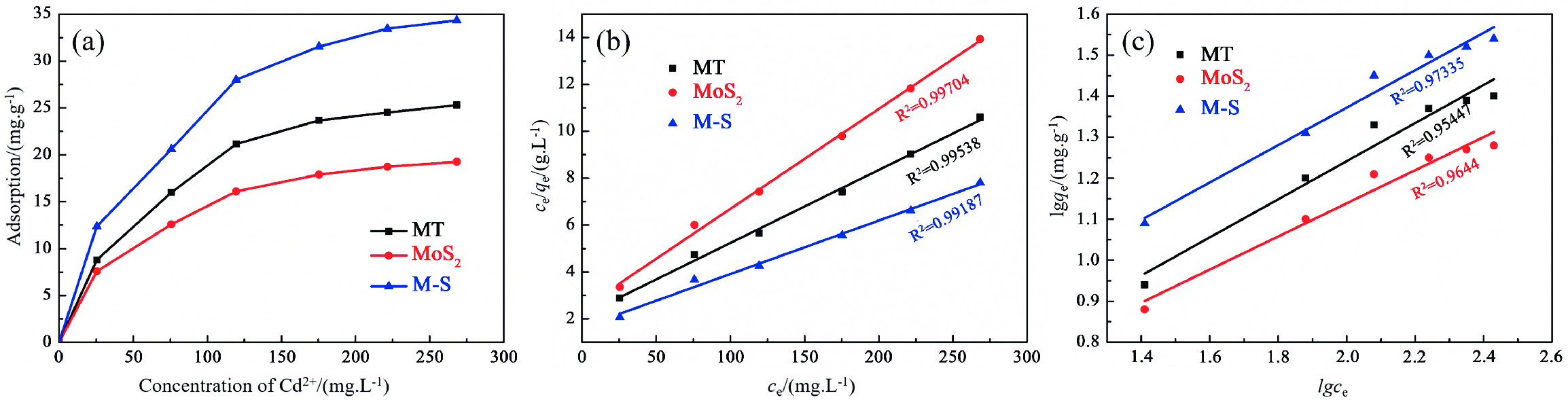
Table 2. Adsorption isotherm parameters of Cd2+ by adsorbents
材料 Langmuir 模型 Freundlich 模型 qm/(mg·g−1) KL/(L·mg−1) R2 1/n KF/(mg·g−1) R2 MT 32.1 0.01468 0.99538 0.57751 1.33748 0.95447 MoS2 23.5 0.01754 0.99704 0.56233 1.56736 0.9644 M−S 43.9 0.01396 0.99187 0.39623 4.20407 0.97335 表 3 蒙脱石基吸附材料对Cd2+的吸附性能对比
Table 3. Comparison of Cd2+ adsorption properties of montmorillonite based adsorbents
吸附剂 Cd2+吸附量/(mg·g−1) 参考文献 蒙脱石−钢渣复合材料 9.76 24 改性蒙脱石 16.54 25 活性炭/钛柱撑蒙脱石 27.97 26 针铁矿−蒙脱石复合材料 19.95 27 碳改性铝柱蒙脱石 28.0 28 活性炭复合钛柱撑蒙脱石 27.5 29 蒙脱石/1T−MoS2复合材料 43.9 本工作 -
[1] 董颖博, 林海, 李冰. 环境矿物材料[M]. 北京: 冶金工业出版社, 2020.
DONG Y B, LIN H, LI B. Environmental mineral material[M]. Beijing: Metallurgical Industry Press, 2020.
[2] 鲁安怀. 天然铁的硫化物净化含铬污水的新方法[J]. 地学前缘, 1998, 5(1/2): 342.
LU A H. A new method for purification of chromium−containing wastewater by natural iron sulfide[J]. Geoscience Frontiers, 1998, 5(1/2): 342.
[3] 石俊仙, 鲁安怀, 陈洁. 天然黄铁矿除Cr(Ⅵ)中Cr2S3物相的发现[J]. 岩石矿物学杂志, 2005, 24(6): 539−542.
SHI J X, LU A H, CHEN J. The discovery of Cr2S3 phas during the treatment of Cr (Ⅵ) by natural pyrite[J]. Acta Petrologica et Mineralogica, 2005, 24(6): 539−542.
[4] 修其慧. 过渡金属氧硫化物及其复合材料去除重金属离子的吸附机制研究[D]. 青岛: 青岛科技大学, 2019.
XIU Q H. Study on adsorption mechanism of transition metal oxide/sulfide and their composite materials for removal of heavy metal ions[D]. Qingdao: Qingdao University of Science and Technology, 2019.
[5] 张翔. 一种新型硫化物重金属捕集剂的合成与应用[J]. 广州化工, 2014, 42(19): 130−132.
ZHANG X. The synthesis and application of a kind of sulfide for wastewater with heavy metal lons treatment[J]. Guangzhou Chemical Industry, 2014, 42(19): 130−132.
[6] 汪辰, 毕磊, 潘纲. 硫化锰纳米颗粒高效去除重金属镉[J]. 环境工程学报, 2020, 14(1): 24−33.
WANG C, BI L, PAN G. High−efficiency removal of heavy metal cadmium by manganese sulfide nanoparticles[J]. Journal of Environmental Engineering, 2020, 14(1): 24−33.
[7] 刘阳, 张新波, 赵樱灿. 二维MoS2纳米材料及其复合物在水处理中的应用[J]. 化学进展, 2020, 32(5): 642−655.
LIU Y, ZHANG X B, ZHAO Y C. Two−dimensional MoS2 nanomaterials and applications in water treatment[J]. Progress in Chemistry, 2020, 32(5): 642−655.
[8] 陈德良, 董会娜, 张锐. 二硫化钼基纳米复合材料的构筑与应用[J]. 郑州大学学报(工学版), 2015, 36(6): 18−29.
CHEN D L, DONG H N, ZHANG R. Advances in synthesis and applications of molybdenum disulfide based nanocomposites[J]. Journal of Zhengzhou University (Engineering Science), 2015, 36(6): 18−29.
[9] 杜淼, 张馨. 二维纳米材料在水处理中的应用研究进展[J]. 无机盐工业, 2020, 52(1): 17−21.
DU M, ZHANG X. Progress in application research of two−dimensional nanomaterials in water treatment[J]. Inorganic Chemicals Industry, 2020, 52(1): 17−21.
[10] LI Z, FAN R, HUA Z, et al. Ethanol introduced synthesis of ultrastable 1T−MoS2 for removal of Cr(Ⅵ)[J]. Journal of Hazardous Materials, 2020, 394: 122525. doi: 10.1016/j.jhazmat.2020.122525
[11] WANG Z, SIM A, URBAN J J, et al. Removal and recovery of heavy metal ions by two−dimensional MoS2 nanosheets: performance and mechanisms[J]. Environmental Science & Technology, 2018, 52: 9741−9748.
[12] KANG Y, NAJMAEI S, LIU Z, et al. Plasmonic hot electron induced structural phase transition in a MoS2 monolayer[J]. Advanced Materials, 2014, 26: 6467−6471. doi: 10.1002/adma.201401802
[13] YIN Y, HAN J, ZHANG Y, et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets[J]. Journal of the American Chemical Society, 2016, 138: 7965−7972. doi: 10.1021/jacs.6b03714
[14] LIU W, XU S, GUAN S, et al. Confined synthesis of carbon nitride in a layered host matrix with unprecedented solid−state quantum yield and stability[J]. Advanced Materials, 2018, 30(2): 1704376.1-1704376.8.
[15] LI Z, ZHANG X, CHENG H, et al. Confined synthesis of 2D nanostructured materials toward electrocatalysis[J]. Advanced Energy Materials, 2020(11):10. DOI:10.1002/aenm.201900486.
[16] CHEN X, WANG Z, WEI Y, et al. High phase−purity 1T−MoS2 ultrathin nanosheets by a spatially confined template[J]. Angewandte Chemie International Edition, 2019, 131: 17785−17788.
[17] 陈方明, 陆琦. 天然矿物材料在废水处理中的应用[J]. 化工矿产地质, 2004, 26(1): 35−40.
CHEN F M, LU Q. The application of natural mineral materials to the disposal of wastewater[J]. Geology of Chemical Minerals, 2004, 26(1): 35−40.
[18] 陈尧, 马玉龙, 谢丽, 等. 有机蒙脱石的制备及其应用研究进展[J]. 材料导报, 2009, 23(14): 432−434+438.
CHEN Y, MA Y L, XIE L, et al. Development of preparation and application of organic−montmorillonite[J]. Materials Reports, 2009, 23(14): 432−434+438.
[19] YANG X, ZHU H, LIU J, et al. A mesoporous structure for efficient photocatalysts: Anatase nanocrystals attached to leached clay layers[J]. Microporous and Mesoporous Materials, 2008, 112: 32−44. doi: 10.1016/j.micromeso.2007.09.017
[20] PENG K, FU L, YANG H, et al. Hierarchical MoS2 intercalated clay hybrid nanosheets with enhanced catalytic activity[J]. Nano Research, 2017, 10: 570−583. doi: 10.1007/s12274-016-1315-3
[21] WANG Z, SIM A, URBAN J J, et al. Removal and recovery of heavy metal ions by two−dimensional MoS2 nanosheets: Performance and mechanisms[J]. Environmental Science & Technology, 2018, 52(17): 9741−9748.
[22] ZHAN W, YUAN Y, ZHANG X, et al, Efficient and selective Lead (Ⅱ) removal within a wide concentration range through chemisorption and electrosorption coupling process via defective MoS2 electrode[J]. Separation and Purification Technology, 2024, 329: 125183.
[23] 夏银, 刘月迎, 王丽娟, 等. 蛭石对水中重金属离子的吸附性能[J]. 硅酸盐学报, 2022, 50(5): 1357−1363.
XIA Y, LIU Y Y, WANG L J, et al. Adsorption properties of heavy metal ions in water by vermiculite[J]. Journal of the Chinese Ceramic Society, 2022, 50(5): 1357−1363.
[24] 来雪慧, 闫彩, 任晓莉, 等. 蒙脱石−钢渣复合吸附颗粒对水中Cd2+的吸附及其它离子的影响研究[J]. 中北大学学报(自然科学版, 2018, 39(5): 566−571.
LAI X H, YAN C, REN X L, et al. Research on Cd2+ adsorption and effects of heavy ions of montmorillonite−slag composite particle[J]. Journal of North University of China (Natural Science Edition), 2018, 39(5): 566−571.
[25] 江湛如, 黄放, 张攀, 等. 两种天然矿物改性材料对模拟废水中Cd2+的吸附特性[J]. 环境科学研究, 2019, 32(2): 356−364.
JIANG Z R, HUANG F, ZHANG P, et al. Adsorption characteristics of cadmium(Ⅱ) simulated wastewater by two organic modified mineral materials[J]. Research of Environmental Sciences, 2019, 32(2): 356−364.
[26] 赵徐霞, 钛柱撑蒙脱石复合吸附剂的制备及对Cd2+的吸附研究[D]. 贵阳: 贵州大学, 2019.
ZHAO X X. The preparation of activated carbon/titanium pillared montmorillonite composite adsorbent and adsorption of cadmium ions[D]. Guiyang: Guizhou University, 2019.
[27] 刘琦, 任帮政, 张是洲, 等. Cd2+在针铁矿蒙脱石及铁−蒙复合物上的吸附特性[J], 现代盐化工. 2021, 48(2): 41−43.
LIU Q, REN B Z, ZHANG S Z, et al. Adsorption characteristics of Cd2+ on goethite−montmorillonite complexes[J]. Modern Salt and Chemical Industry, 2021, 48(02): 41−43.
[28] YU R, WANG S, WANG D, et al. Removal of Cd2+ from aqueous solution with carbon modified aluminum−pillared montmorillonite[J]. Catalysis Today, 2008, 139: 135−139. doi: 10.1016/j.cattod.2008.08.015
[29] ZHAO X, TUO B, LONG S, et al. Study on adsorption of Cd2+ by Ti−pillared montmorillonite−mixed activated carbon[J]. Micro & Nano Letters, 2021, 16(5): 304−312.
-



 下载:
下载: