Mineralogical Characteristics of Two Clay-type Lithium Resources in Yuxi, China, and Nevada, the United States of America
-
摘要:
中国云南玉溪和美国内华达均已发现大量黏土型锂资源,目前关于两地区样品矿物学特征的研究相对不足,而对黏土型锂资源进行充分的矿物学特征研究是锂提取工作开展的重要前提。本文采用X射线荧光光谱、电感耦合等离子体质谱、X射线粉晶衍射、扫描电镜等分析技术,从化学组成、矿物组成、微观形貌等角度对云南玉溪的两个黏土型锂资源样品(YM-1和YM-2)和美国内华达的两个黏土型锂资源样品(Ame-1和Ame-2)进行对比分析。结果表明:玉溪地区和内华达地区样品的锂含量均高于1000μg/g,具有一定的开发利用价值,但这两个地区的黏土型锂资源样品在主要化学成分、矿物组成、微观形貌和锂赋存状态四个方面均存在较大差异。具体来说,①主要化学成分差异:玉溪地区样品的主要化学成分为SiO2和Al2O3(硅、铝氧化物总量超过80%),而内华达地区样品的主要化学成分为SiO2(60.39%)或CaO(42.30%)。②矿物组成差异:玉溪地区样品的主要矿物为高岭石和蒙脱石,而内华达地区样品的主要矿物为石英、绿脱石、斯皂石或方解石。③微观形貌差异:玉溪地区样品是由表面平坦、边缘圆滑且大小相对均一的片层状结构堆叠而成,而内华达地区样品主要表现为大小不一的块状矿物聚集体。④锂赋存状态差异:玉溪地区样品中的锂主要赋存于蒙脱石中,而内华达地区样品中的锂主要赋存于蒙皂石族矿物中。本研究结果基本明确了云南玉溪地区和美国内华达地区黏土型锂资源的矿物学特征,可为这两个地区黏土型锂资源后期的开发利用提供科学依据。
-
关键词:
- 黏土型锂资源 /
- 矿物学特征 /
- X射线荧光光谱法 /
- 电感耦合等离子体质谱法 /
- X射线粉晶衍射法
Abstract:BACKGROUND Sufficient mineralogical research on clay-type lithium resources is an important prerequisite for lithium extraction and leaching. Numerous clay-type lithium resources have been discovered in both Yuxi City of Yunnan Province in China and the state of Nevada in the USA; however, existing research on their mineralogical characteristics is relatively insufficient.
OBJECTIVES To explore the main chemical composition, phase composition, microscopic morphology, Li occurrence and other characteristics of clay-type lithium resource samples from Yuxi and Nevada and to provide theoretical support for the extraction and leaching of clay-type lithium resources in these two areas.
METHODS X-ray fluorescence spectroscopy, inductively coupled plasma emission spectroscopy, inductively coupled plasma mass spectrometry, powder crystal X-ray diffraction analysis, and scanning electron microscopy were used to analyze the mineral and chemical differences in the clay-type lithium resources between the two samples (YM-1 and YM-2) collected from Yuxi City, Yunnan Province, and the two samples (Ame-1 and Ame-2) from Nevada, USA.
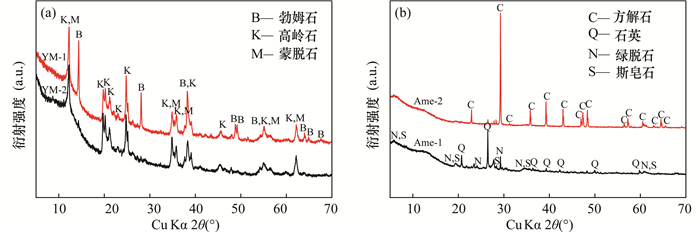
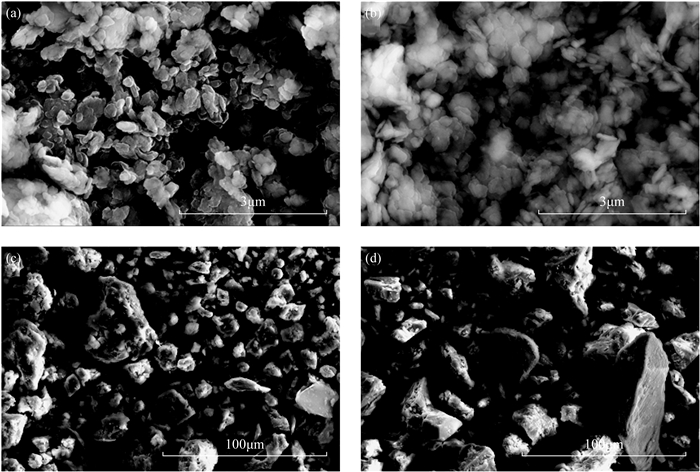
RESULTS The lithium contents of YM-1 and YM-2 and Ame-1 and Ame-2 were higher than 1000μg/g, which exhibited a certain development and utilization value. However, the clay-type lithium resource samples from the two investigated regions showed large differences in chemical composition, mineral composition, microscopic morphology, and lithium occurrences. (1) YM-1 and YM-2 had similar SiO2 and Al2O3 content, with the total amount of silicon and aluminum oxides exceeding 80%, whereas Ame-1 contained 60.39% SiO2 and Ame-2 comprised 42.30% CaO. (2) YM-1 and YM-2 were composed of kaolinite and montmorillonite, whereas Ame-1 and Ame-2 were composed of quartz, nontronite, stevensite, or calcite. (3) YM-1 and YM-2 were stacked in a layered structure with flat surfaces and had round edges and a relatively uniform size, whereas Ame-1 and Ame-2 were mainly represented by massive mineral aggregates of different sizes. (4) Montmorillonite in YM-1 and YM-2 served as the lithium source, whereas lithium in Ame-1 and Ame-2 originated from smectite minerals or illite.
CONCLUSIONS This study elucidated the mineralogical characteristics of clay-type lithium resources in Yuxi (Yunnan, China) and Nevada (USA). It provides a scientific basis for future development and utilization of the clay-type lithium resources in these two regions.
-

-
表 1 黏土型锂资源样品的主要化学组成
Table 1. Main chemical composition of clay-type lithium deposit samples
样品来源 样品编号 含量(%) SiO2 Al2O3 Fe2O3 K2O Na2O MgO CaO TiO2 P2O5 SO3 LOI 中国云南玉溪地区 YM-1 31.97 49.30 1.57 0.57 0.09 0.25 0.08 1.87 0.02 0.02 13.56 YM-2 42.96 38.99 0.83 1.66 0.15 0.37 0.13 2.15 nd nd 12.17 美国内华达地区 Ame-1 60.39 9.25 3.69 3.15 2.03 11.15 3.79 0.55 0.17 0.01 5.03 Ame-2 15.20 1.17 0.39 0.33 0.35 4.84 42.30 0.07 0.02 nd 34.35 表 2 黏土型锂资源样品微量元素含量
Table 2. Trace elements content of clay-type lithium deposit samples
样品来源 样品编号 含量(μg/g) Li Ba Cr Zr V Sr Mn P Ga Nb Ni ΣREE 中国云南玉溪地区 YM-1 1000 414 580 561 342 43.3 14 80 85.2 45.5 50.2 180.54 YM-2 1260 432 320 581 135 12.6 7 30 35.3 52.1 24.8 150.89 美国内华达地区 Ame-1 1940 435 151 235 52 1165 367 790 15.1 13.8 60.5 153.15 Ame-2 1060 83.7 13 24 8 1920 111 120 1.9 1.8 7.1 57.41 -
[1] 焦距, 杨啸涛, 袁继海, 等. 便携式Li-K分析仪的研制及其在锂辉石中锂的分析应用[J]. 岩矿测试, 2016, 35(4): 366-372. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.04.005
Jiao J, Yang X T, Yuan J H, et al. Development of a portable Li-K analyzer and its application in the determination of lithium in spodumene[J]. Rock and Mineral Analysis, 2016, 35(4): 366-372. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.04.005
[2] Xing P, Wang C Y, Zeng L, et al. Lithium extraction and hydroxysodalite zeolite synthesis by hydrothermal conversion of α-spodumene[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10): 9498-9505. http://www.researchgate.net/publication/332885942_Lithium_Extraction_and_Hydroxysodalite_Zeolite_Synthesis_by_Hydrothermal_Conversion_of_a-Spodumene
[3] Gao L, Wang H Y, Li J L, et al. Recovery of lithium from lepidolite by sulfuric acid and separation of Al/Li by nanofiltration[J]. Minerals, 2020, 10(11): 981. doi: 10.3390/min10110981
[4] 于沨, 王登红, 于扬, 等. 国内外主要沉积型锂矿分布及勘查开发现状[J]. 岩矿测试, 2019, 38(3): 354-364. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201901180013
Yu F, Wang D H, Yu Y, et al. The distribution and exploration status of domestic and foreign sedimentary-type lithium deposits[J]. Rock and Mineral Analysis, 2019, 38(3): 354-364. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201901180013
[5] Kesler S E, Gruber P W, Medina P A, et al. Global lithium resources: Relative importance of pegmatite, brine and other deposits[J]. Ore Geology Reviews, 2012, 48: 55-69. doi: 10.1016/j.oregeorev.2012.05.006
[6] Benson T R, Coble M A, Rytuba J J, et al. Lithium enrichment in intracontinental rhyolite magmas leads to Li deposits in Caldera basins[J]. Nature Communications, 2017, 8: 270. doi: 10.1038/s41467-017-00234-y
[7] An J W, Kang D J, Tran K T, et al. Recovery of lithium from Uyuni salar brine[J]. Hydrometallurgy, 2012, 117-118: 64-70. doi: 10.1016/j.hydromet.2012.02.008
[8] 刘卓, 周云峰, 柴登鹏, 等. 从盐湖卤水中提取锂的技术研究进展与展望[J]. 材料导报, 2015, 29(增刊2): 133-137. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB2015S2033.htm
Liu Z, Zhou Y F, Chai D P, et al. Progress and prospects of lithium extraction technology from salt lake brine[J]. Materials Review, 2015, 29(Supplement 2): 133-137. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB2015S2033.htm
[9] Song Y F, Zhao T Y, He L H, et al. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate[J]. Hydrometallurgy, 2019, 189: 105141. doi: 10.1016/j.hydromet.2019.105141
[10] Rosales G D, Resentera A C J, Gonzalez J A, et al. Efficient extraction of lithium from β-spodumene by direct roasting with NaF and leaching[J]. Chemical Engineering Research and Design, 2019, 150: 320-326. doi: 10.1016/j.cherd.2019.08.009
[11] 苏慧, 朱兆武, 王丽娜, 等. 矿石资源中锂的提取与回收研究进展[J]. 化工学报, 2019, 70(1): 10-23. https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201901002.htm
Su H, Zhu Z W, Wang L N, et al. Research progress in extraction and recovery of lithium from hard-rock ores[J]. Journal of Chemical Industry and Engineering, 2019, 70(1): 10-23. https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201901002.htm
[12] Guo H, Yu H Z, Zhou A A, et al. Kinetics of leaching lithium from α-spodumene in enhanced acid treatment using HF/H2SO4 as medium[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(2): 407-415. doi: 10.1016/S1003-6326(19)64950-2
[13] 温汉捷, 罗重光, 杜胜江, 等. 碳酸盐黏土型锂资源的发现及意义[J]. 科学通报, 2020, 65(1): 53-59. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB202001009.htm
Wen H J, Luo C G, Du S J, et al. Carbonate-hosted clay-type lithium deposit and its prospecting significance[J]. Chinese Science Bulletin, 2020, 65(1): 53-59. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB202001009.htm
[14] 宋云华, 沈丽璞, 张乃娴, 等. 河南某黏土矿(岩)中黏土矿物及其稀土、锂等元素的初步研究[J]. 中国科学(化学), 1987(2): 204-213. http://www.cnki.com.cn/Article/CJFDTotal-JBXK198702011.htm
Song Y H, Shen L P, Zhang N X, et al. Preliminary study on clay minerals, rare earth, lithium and other elements in a clay ore (rock) in Henan[J]. Scientia Sinica (Chimica), 1987(2): 204-213. http://www.cnki.com.cn/Article/CJFDTotal-JBXK198702011.htm
[15] Amer A M. The hydrometallurgical extraction of lithium from Egyptian montmorillonite-type clay[J]. JOM, 2008, 60(10): 55-57. doi: 10.1007/s11837-008-0137-5
[16] 李荣改, 宋翔宇, 高志, 等. 河南某地低品位含锂黏土矿提锂新工艺研究[J]. 矿冶工程, 2014, 34(6): 81-84. doi: 10.3969/j.issn.0253-6099.2014.06.020
Li R G, Song X Y, Gao Z, et al. New technology for extracting Li from low-grade lithium-bearing clay[J]. Mining and Metallurgical Engineering, 2014, 34(6): 81-84. doi: 10.3969/j.issn.0253-6099.2014.06.020
[17] Swain B. Recovery and recycling of lithium-A review[J]. Separation and Purification Technology, 2017, 172: 388-403. doi: 10.1016/j.seppur.2016.08.031
[18] Gu H N, Guo T F, Wen H J, et al. Leaching efficiency of sulfuric acid on selective lithium leachability from bauxitic claystone[J]. Minerals Engineering, 2020, 145: 106076. doi: 10.1016/j.mineng.2019.106076
[19] 朱丽, 杨永琼, 顾汉念, 等. 黏土型锂资源中锂的浸出试验[J]. 有色金属(冶炼部分), 2020(11): 35-40. doi: 10.3969/j.issn.1007-7545.2020.11.007
Zhu L, Yang Y Q, Gu H N, et al. Study on leaching of lithium from clay-type lithium deposit[J]. Nonferrous Metals (Extractive Metallurgy), 2020(11): 35-40. doi: 10.3969/j.issn.1007-7545.2020.11.007
[20] Edmunnd V E. Lime-gypsum processing of McDermitt clay for lithium recovery[R]. US: Bureau of Mines, 1983.
[21] Crocker L, Lien R H. Lithium and its recovery from low-grade Nevada clays[R]. US: Bureau of Mines, Department of Interior, 1987.
[22] Swain B. Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: A review[J]. Journal of Chemical Technology and Biotechnology, 2016, 91(10): 2549-2562. doi: 10.1002/jctb.4976
[23] Castor S B, Henry C D. Lithium-rich claystone in the McDermitt Caldera, Nevada, USA: Geologic, mineralogical, and geochemical characteristics and possible origin[J]. Minerals, 2020, 10(1): 68. doi: 10.3390/min10010068
[24] Glanzman R K, Mccarthy J H, Rytuba J J. Lithium in the McDermitt Caldera, Nevada and Oregon[J]. Energy, 1978, 3(3): 347-353. doi: 10.1016/0360-5442(78)90031-2
[25] 贾玉衡, 钱建平. 电子探针-电感耦合等离子体质谱法研究不同种类石榴石的稀土元素配分和矿物学特征[J]. 岩矿测试, 2020, 39(6): 886-895. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.202005060007
Jia Y H, Qian J P. Study on REE distribution and mineralogical characteristics of different garnets by electron probe and inductively coupled plasma-mass spectrometry[J]. Rock and Mineral Analysis, 2020, 39(6): 886-895. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.202005060007
[26] 洪汉烈, 方谦, 王朝文, 等. 岩浆母质对蚀变黏土矿物的约束: 以贵州新民剖面P-T界线附近火山灰层为例[J]. 地球科学, 2017, 42(2): 161-172. doi: 10.3969/j.issn.1672-6561.2017.02.003
Hong H L, Fang Q, Wang C W, et al. Constraints of parent magma on altered clay minerals: A case study on the ashes near the Permin-Triassic boundary in Xinmin Section, Guizhou Province[J]. Earth Science, 2017, 42(2): 161-172. doi: 10.3969/j.issn.1672-6561.2017.02.003
[27] 孙海平, 蒋婷, 李祉贤. 关于高岭土矿物的岩石学特征研究[J]. 科学技术创新, 2016(4): 80-82. doi: 10.3969/j.issn.1673-1328.2016.04.073
Sun H P, Jiang T, Li Z X. Research on petrological characteristics of kaolin minerals[J]. Scientific and Technological Innovation, 2016(4): 80-82. doi: 10.3969/j.issn.1673-1328.2016.04.073
[28] Shi D, Zhang L C, Peng X W, et al. Extraction of lithium from salt lake brine containing boron using multistage centrifuge extractors[J]. Desalination, 2018, 441: 44-51. doi: 10.1016/j.desal.2018.04.029
[29] 徐萍, 钱晓明, 郭昌盛, 等. 用于盐湖卤水镁锂分离的纳滤技术研究进展[J]. 材料导报, 2019, 33(3): 410-417. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201903007.htm
Xu P, Qian X M, Guo C S, et al. Nanofiltration technology used for separation of magnesium and lithium from salt lake brine: A survey[J]. Materials Reports, 2019, 33(3): 410-417. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201903007.htm
[30] Ashraf M A, Li X C, Wang J F, et al. DiaNanofiltration-based process for effective separation of Li+ from the high Mg2+/Li+ ratio aqueous solution[J]. Separation and Purification Technology, 2020, 247: 116965. doi: 10.1016/j.seppur.2020.116965
[31] Li Z, Binnemans K. Selective removal of magnesium from lithium-rich brine for lithium purification by synergic solvent extraction using beta-diketones and Cyanex 923[J]. Aiche Journal, 2020, 66(7): e16246. http://www.researchgate.net/publication/340606601_Selective_removal_of_magnesium_from_lithium-rich_brine_for_lithium_purification_by_synergic_solvent_extraction_using_-diketones_and_Cyanex_923
[32] 戴江洪, 王宏岩, 李平. 高纯碳酸锂制备研究进展[J]. 中国有色冶金, 2020, 49(1): 49-53. doi: 10.3969/j.issn.1672-6103.2020.01.013
Dai J H, Wang H Y, Li P. Research development of preparation of high purity lithium carbonate[J]. China Nonferrous Metallurgy, 2020, 49(1): 49-53. doi: 10.3969/j.issn.1672-6103.2020.01.013
[33] Choubey P K, Kim M S, Srivastava R R, et al. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part Ⅰ: From mineral and brine resources[J]. Minerals Engineering, 2016, 89: 119-137. doi: 10.1016/j.mineng.2016.01.010
[34] Reig M, Casas S, Gibert O, et al. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals[J]. Desalination, 2016, 382: 13-20. doi: 10.1016/j.desal.2015.12.013
[35] Bi Q, Zhang Z, Zhao C, et al. Study on the recovery of lithium from high Mg2+/Li+ ratio brine by nanofiltration[J]. Water Science and Technology, 2014, 70(10): 1690-1694. doi: 10.2166/wst.2014.426
[36] 崔燚, 罗重光, 徐林, 等. 黔中九架炉组富锂黏土岩系的风化成因及锂的富集规律[J]. 矿物岩石地球化学通报, 2018, 37(4): 696-704. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH201804014.htm
Cui Y, Luo C G, Xu L, et al. Weathering origin and enrichment of lithium in clay rocks of the Jiujialu Formation, central Guizhou Province, southwest China[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2018, 37(4): 696-704. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH201804014.htm
[37] 赵艺森, 王海芳, 魏阳. 赤泥的综合利用研究进展[J]. 现代化工, 2019, 39(3): 55-58. https://www.cnki.com.cn/Article/CJFDTOTAL-XDHG201903012.htm
Zhao Y S, Wang H F, Wang Y. Advances in comprehensive utilization of red mud[J]. Modern Chemical Industry, 2019, 39(3): 55-58. https://www.cnki.com.cn/Article/CJFDTOTAL-XDHG201903012.htm
[38] 宁树正, 黄少青, 朱士飞, 等. 中国煤中金属元素成矿区带[J]. 科学通报, 2019, 64(4): 45-57. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB201924006.htm
Ning S Z, Huang S Q, Zhu S F, et al. Mineralization zoning of coal-metal deposits in China[J]. Chinese Science Bulletin, 2019, 64(4): 45-57. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB201924006.htm
[39] 何涌, 雷新荣. 结晶化学[M]. 北京: 化学工业出版社, 2008.
He Y, Lei X R. Crystal chemistry[M]. Beijing: Chemical Industry Press, 2008.
[40] 赵晨, 印万忠, 朱一民, 等. 方解石与硅灰石可浮性差异的机理研究[J]. 矿产保护与利用, 2020(4): 82-88. https://www.cnki.com.cn/Article/CJFDTOTAL-KCBH202004015.htm
Zhao C, Yin W Z, Zhu Y M, et al. Study on the mechanism of floatability differences between calcite and wollastonite[J]. Conservation and Utilization of Mineral Resources, 2020(4): 82-88. https://www.cnki.com.cn/Article/CJFDTOTAL-KCBH202004015.htm
[41] Song X W, Cao Y W, Bu X Z, et al. Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process[J]. Applied Surface Science, 2021, 536: 147958. doi: 10.1016/j.apsusc.2020.147958
[42] 卢龙飞, 蔡进功, 刘文汇, 等. 泥岩与沉积物中黏土矿物吸附有机质的三种赋存状态及其热稳定性[J]. 石油与天然气地质, 2013(1): 16-26. https://d.wanfangdata.com.cn/periodical/syytrqdz201301003
Lu L F, Cai J G, Liu W H, et al. Occurrence and thermostability of absorbed organic matter on clay minerals in mudstones and muddy sediments[J]. Oil & Gas Geology, 2013(1): 16-26. https://d.wanfangdata.com.cn/periodical/syytrqdz201301003
[43] 牛庆合. 超临界CO2注入无烟煤力学响应机理与可注性试验研究[D]. 徐州: 中国矿业大学, 2019.
Niu Q H. Experimental study on the mechanical response mechanism and injectivity with supercritical CO2 injection in anthracite[D]. Xuzhou: China University Mining and Technology, 2019.
[44] 李荣改, 宋翔宇, 徐靖, 等. 河南某含锂黏土矿工艺矿物学研究[J]. 矿产保护与利用, 2014(6): 38-41.
Li R G, Song X Y, Xu J, et al. Process mineralogy research on a lithium clay in Henan[J]. Conservation and Utilization of Mineral Resources, 2014(6): 38-41.
[45] 张俊文. 花岗岩风化过程锂同位素行为及其环境指示意义[D]. 武汉: 中国地质大学(武汉), 2018.
Zhang J W. Behavior of lithium isotopes and environmental indications during granite weathering[D]. Wuhan: China University of Geosciences (Wuhan), 2018.
[46] 钟海仁, 孙艳, 杨岳清, 等. 铝土矿(岩)型锂资源及其开发利用潜力[J]. 矿床地质, 2019, 38(4): 898-916. https://www.cnki.com.cn/Article/CJFDTOTAL-KCDZ201904014.htm
Zhong H R, Sun Y, Yang Y Q, et al. Bauxite (aluminum)-type lithium resources and analysis of its development and utilization potential[J]. Mineral Deposits, 2019, 38(4): 898-916. https://www.cnki.com.cn/Article/CJFDTOTAL-KCDZ201904014.htm
-




 下载:
下载: