Acid Leaching Performance of Gibbsite-type Bauxite with High Iron Content
-
摘要:
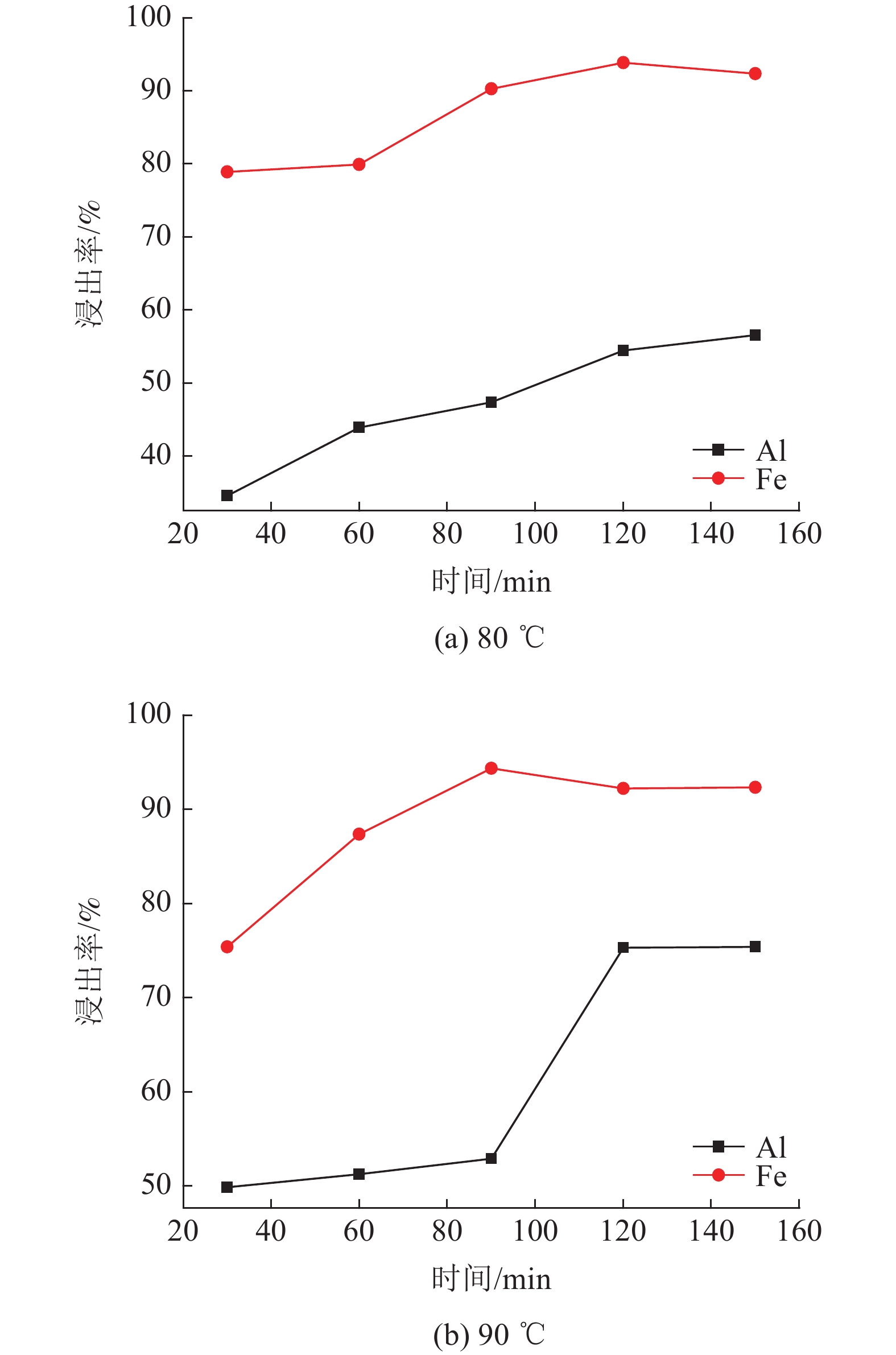
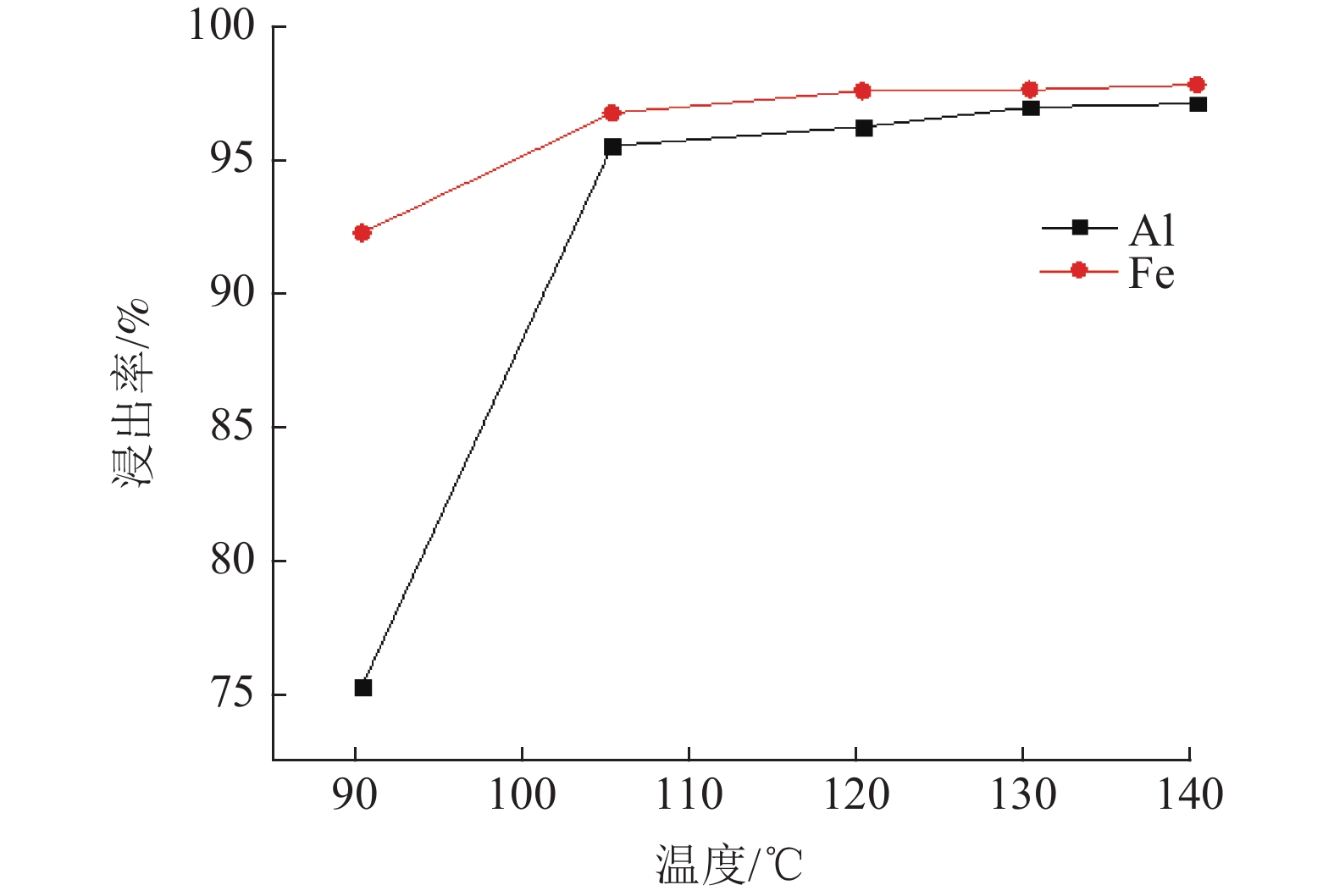
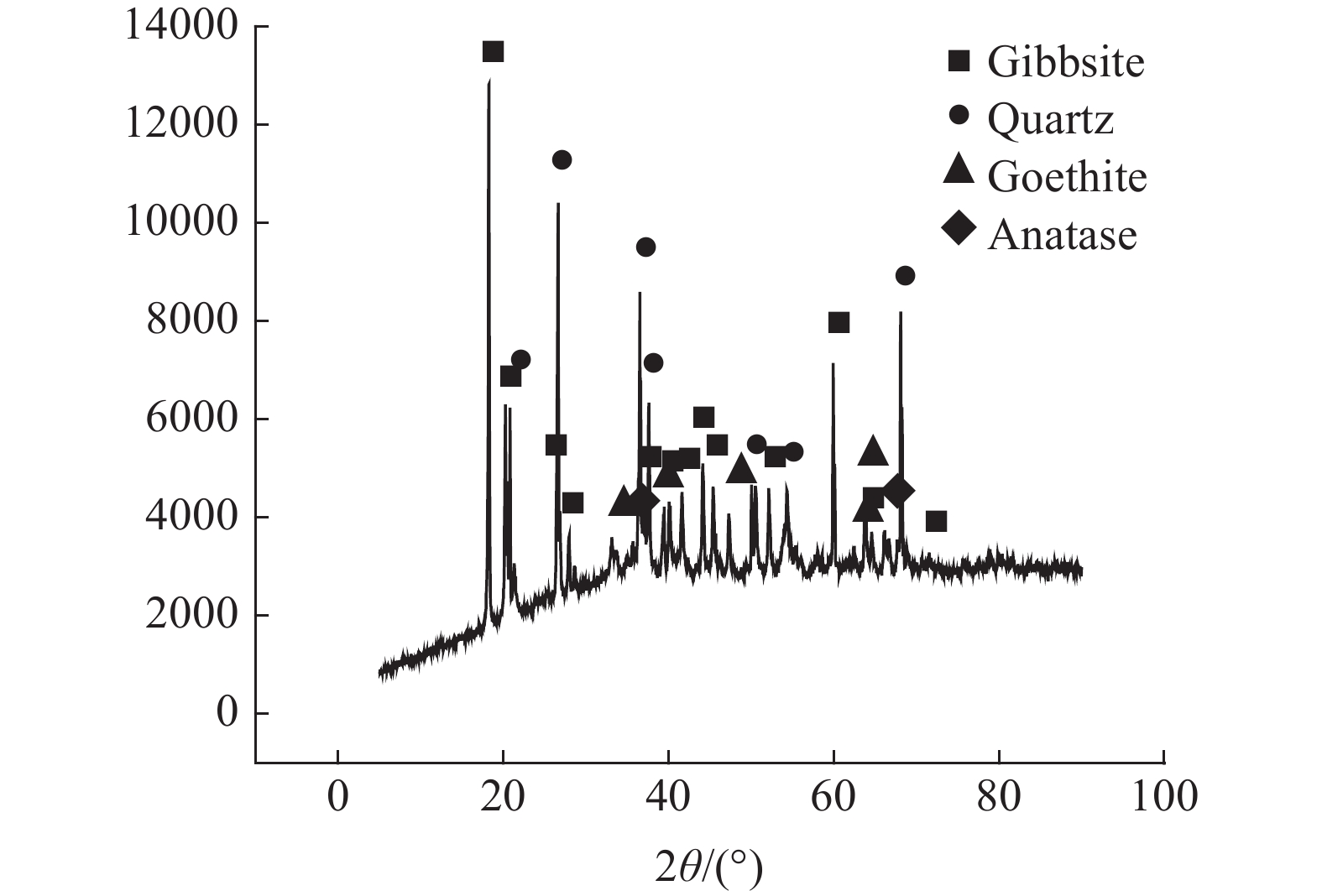
对于铝硅比低的高铁铝土矿采用拜耳法已不能经济地提取。以国外某低品位三水铝石矿为研究对象,采用盐酸为浸出介质对其进行酸浸性能研究。主要考察矿石粒度、液固比及时间和温度对浸出效果的影响。结果表明:温度对铝元素浸出效果影响显著。当矿石粒度55 μm,液固比100∶7,温度105℃,时间120 min,采用密闭加压方式浸出,此时铝元素和铁元素的浸出率均高于95%,均得到有效浸出,与浸出渣产物表征一致;对该高铁三水矿酸浸过程进行动力学研究表明,铁和铝的酸浸过程均受内扩散控制。
Abstract:High-iron bauxite with low A/S ratio cannot be extracted economically by Bayer process. Taking a low-grade gibbsite ore abroad as the research object, the acid leaching performance of gibbsite was studied by using hydrochloric acid as leaching medium. The effects of ore particle size, liquid-solid ratio, time and temperature on leaching efficiency were investigated. The results show that temperature has a significant effect on the leaching effect of aluminum. When the particle size of ore is 55 μm, the ratio of liquid to solid is 100:7, the temperature is 105℃, and the time is 120 min, the leaching rate of aluminum and iron are both higher than 95%, and they are effectively leached. It is consistent with the characterization of leaching residue. The kinetics of the acid leaching process of the high iron gibbsite ore shows that the acid leaching process of iron and aluminum is controlled by internal diffusion.
-
Key words:
- Acid leaching /
- Bauxite /
- Alumina
-

-
表 1 三水铝石矿的化学成分 /%
Table 1. Chemical composition of gibbsite
Al2O3 SiO2 Fe2O3 TiO2 CaO MgO 灼减 38.51 18.58 19.26 1.09 0.05 0.08 22.20 表 2 三水铝石矿的物相成分分析 /%
Table 2. Phase composition analysis of gibbsite
三水铝石 石英 针铁矿 锐钛矿 其他 58.59 18.58 21.43 1.09 0.31 -
[1] 陈燕清. 广西某高硫高铁铝土矿拜耳法溶出试验研究[J]. 矿产综合利用, 2019(2):46-50. doi: 10.3969/j.issn.1000-6532.2019.02.009
CHEN Y Q. Study on the bayer dissolving method for high-sulfur and iron bauxite in Guangxi[J]. Multipurpose Utilization of Mineral Resources, 2019(2):46-50. doi: 10.3969/j.issn.1000-6532.2019.02.009
[2] 刘琼霞, 单志强. 广西某铝土矿Bayer-CaO法溶出试验[J]. 矿产综合利用, 2019(3):27-30. doi: 10.3969/j.issn.1000-6532.2019.03.006
LIU Q X, SHAN Z Q. Tests on the lime-bayer dissolving method for the bauxite from Guangxi Taiping mining area[J]. Multipurpose Utilization of Mineral Resources, 2019(3):27-30. doi: 10.3969/j.issn.1000-6532.2019.03.006
[3] Azof, F I, Vafeias M, Panias D, et al. The leachability of a ternary CaO-Al2O3-SiO2 slag produced from smelting-reduction of low-grade bauxite for alumina recovery[J]. Hydrometallurgy, 2020, 191:1-12.
[4] Wu Y, Bai H, Xin H X, et al. Study on separation of iron and aluminum of high iron bauxite by reduction roasting[J]. Light Metals, 2019(12):15-19.
[5] 肖永丰. 粉煤灰提取氧化铝方法研究[J]. 矿产综合利用, 2020(4):156-162. doi: 10.3969/j.issn.1000-6532.2020.04.027
XIAO Y F. Study on the methods of leaching alumina from fly ash[J]. Multipurpose Utilization of Mineral Resources, 2020(4):156-162. doi: 10.3969/j.issn.1000-6532.2020.04.027
[6] 贾敏, 杨磊. 煤矸石煅烧活化提取氧化铝技术研究[J]. 矿产综合利用, 2020(2):140-144. doi: 10.3969/j.issn.1000-6532.2020.02.025
JIA M, YANG L. Study on technology of alumina extraction from coal gangue activated by calcination[J]. Multipurpose Utilization of Mineral Resources, 2020(2):140-144. doi: 10.3969/j.issn.1000-6532.2020.02.025
[7] Valeev D, Kunilova I, Shoppert A, et al. High-pressure HCl leaching of coal ash to extract Al into a chloride solution with further use as a coagulant for water treatment[J]. Journal of Cleaner Production 2020, 276: 1-15.
[8] Rivera R M, Xakalashe B, Ounoughene G, et al. Selective rare earth element extraction using high-pressure acid leaching of slags arising from the smelting of bauxite residue[J]. Hydrometallurgy 2019, 184: 162-174.
[9] Zhao Y L, Zheng Y J, He H B, et al. Effective aluminum extraction using pressure leaching of bauxite reaction residue from coagulant industry and leaching kinetics study[J]. Journal of Environmental Chemical Engineering 2020, https://doi.org/10.1016/j.jece.2020.104770.
[10] 赵爱春. 酸法处理高铁铝土矿的基础研究[D]. 沈阳: 东北大学, 2013: 14.
ZHAO A C. Basic study on bauxite with high iron content by acid leaching[D]. Shenyang: Northeastern University, 2013: 4.
[11] 赵爱春, 张廷安, 吕国志, 等. 喷雾热解法制备Al2O3粉末[J]. 东北大学学报(自然科学版), 2012, 33(3):401-404. doi: 10.12068/j.issn.1005-3026.2012.03.024
ZHAO A C, ZHANG T A, LV G Z, et al. Preparation of Al2O3 powders by spray pyrolysis deposition[J]. Journal of Northeastern University(Natural Science), 2012, 33(3):401-404. doi: 10.12068/j.issn.1005-3026.2012.03.024
-



 下载:
下载: