Research on Removal of Chromium (VI) from Waste water on Fly Ash Modified with Alkali Washing and Calcium Oxide Calcining Method
-
摘要:
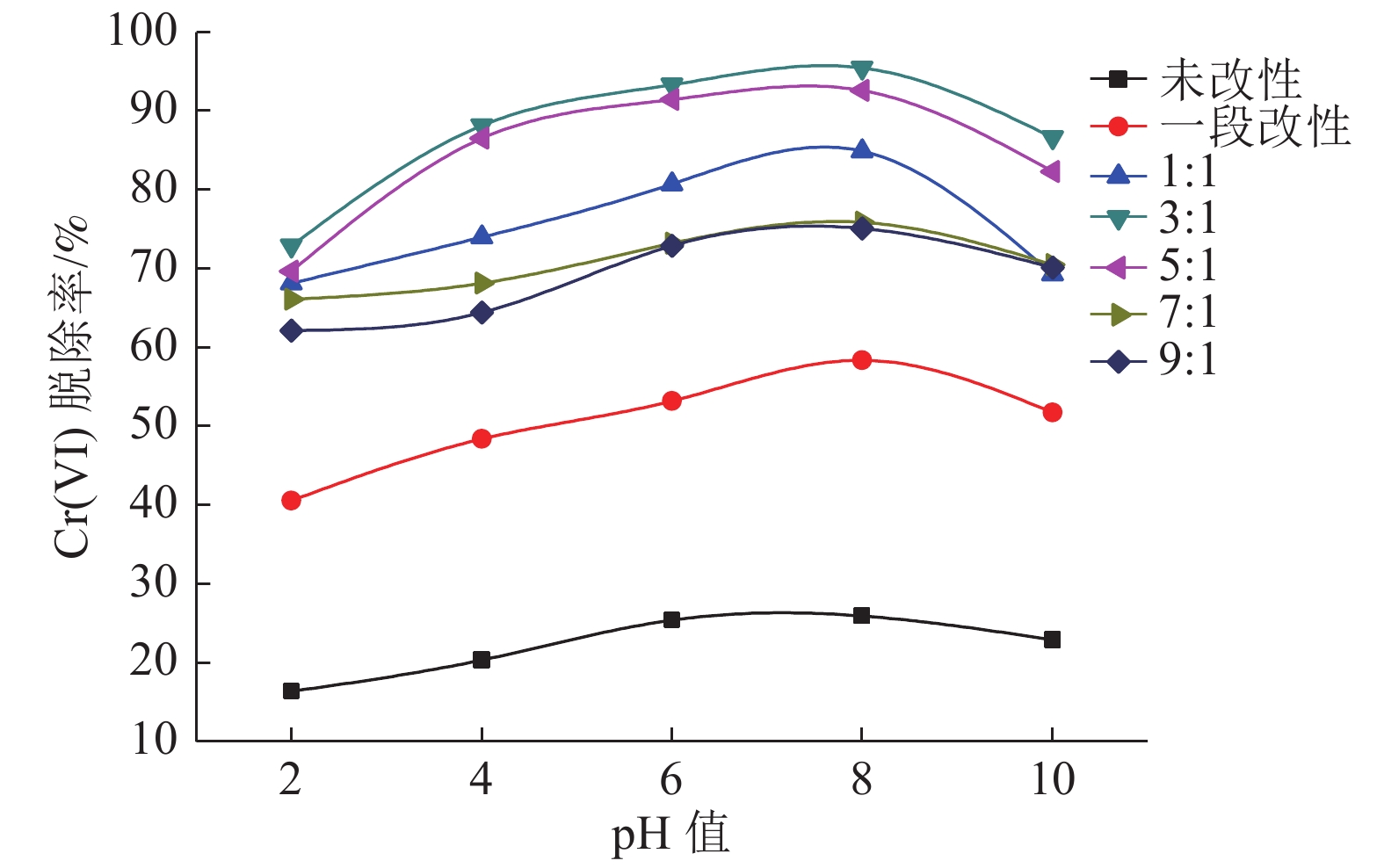
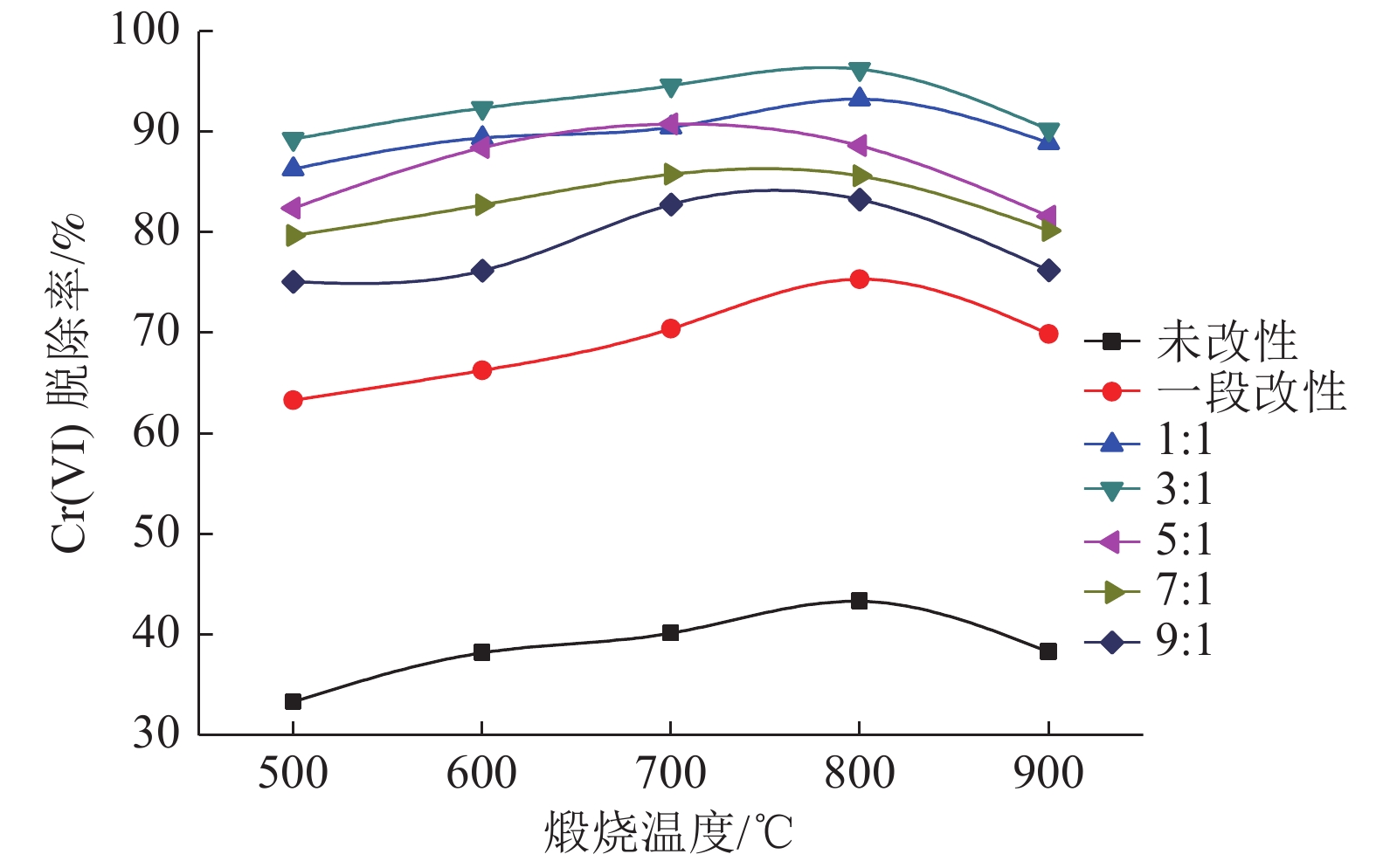
采用碱洗—氧化钙煅烧两段法对粉煤灰进行改性处理,利用红外光谱和扫描电镜对改性前后的粉煤灰的键位基团及形态进行表征,以投加量、pH值、煅烧温度和吸附时间作为变量对含Cr(VI)废水进行吸附处理。结果表明,当改性粉煤灰投加量为6 g/L、废水初始pH值为8、第二段煅烧温度为800℃、粉煤灰与氧化钙配比为3∶1时,吸附容量为16.06 mg/g,吸附效率达96.38%。动力学拟合过程表明该改性粉煤灰对Cr(VI)的吸附符合伪二阶动力学方程,以化学吸附为主,吸附过程具有持续性。
Abstract:Fly ash was modified by the alkali washing and calcium oxide calcining method The infrared spectrometer and scanning electron microscopy were used to characterize the bond groups and morphology before and after modification fly ash. The dosage, pH, calcined temperature and adsorption time were used as variable factors to adsorb Cr (VI)-containing waste water. The results showed that the removal rate of Cr(VI) can reach 96.38% under modified fly ash dosage at 6 g/L, initial pH value at 8, secondary calcination temperature at 800℃ and the ratio with calcium oxide at 3∶1. The adsorption capacity reached 16.06 mg/g. The kinetic fitting process shows that the adsorption of Cr (VI) by the modified fly ash conformed to the pseudo-second-order kinetic equation. This process was dominated by chemical adsorption and continuous.
-

-
表 1 原料粉煤灰的化学组成 /%
Table 1. Chemical composition of raw fly ash
SiO2 Al2O3 CaO MgO Na2O K20 Fe2O3 SO3 53.34 28.53 4.82 1.93 0.62 1.33 5.84 1.57 表 2 粉煤灰改性混合料对Cr(VI)吸附过程的动力学模型拟合参数
Table 2. Kinetic model parameters for Cr(VI) adsorption on modified fly ash mixture
伪一阶动力学模型 伪二阶动力学模型 内颗粒扩散模型 叶洛维奇模型 qe/(mg·g−1) k1/min−1 R2 qe/(mg·g−1) k2/(g·mg−1·min−1) R2 k3 C R2 α/(g·mg−1·min−1) β/(g·mg−1) R2 3.6889 0.037 0.6091 17.09 0.0088 0.9974 0.7126 9.3807 0.7128 15.6753 0.3911 0.8283 -
[1] Miretzky P, Cirelli A F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: a review[J]. Journal of Hazardous Materials, 2010, 180(1):1-19.
[2] Leyva-ramos R, Jacobo-azuara A. Adsorption of chromium(VI) from an aqueous solution on a surfactant-modified zeolite[J]. Colloids & Surface A Physicochemical & Engineering Aspcets, 2008, 330(1):35-41.
[3] 孙霞, 吴益梅, 王琪瑶. 碱溶粉煤灰对废水中六价铬的吸附试验研究[J]. 煤炭科学技术, 2010, 38(10):124-128.
SUN X, WU Y M, WANG Q Y. Adsorption of hexavalent chromium from wastewater by alkali-dissolved fly ash[J]. Coal Science and Technology, 2010, 38(10):124-128.
[4] 范力, 张建强, 程新, 等. 离子交换法及吸附法处理含铬废水的研究进展[J]. 水处理技术, 2009, 35(1):30-33.
FAN L, ZHANG J Q, CHEN X, et al. Research progress in the treatment of chromium containing wastewater by ion exchange and adsorption[J]. Water Treatment Technology, 2009, 35(1):30-33.
[5] Cheng Lang, Liu Hong, Wang Xi, et al. Preparation of CTMAB/attapulgite particles and their adsorption Cr(VI) performance[J]. Environmental science and technology, 2017, 40(10):77-81.
[6] 欧阳平, 范洪勇. 粉煤灰吸附剂的研究现状[J]. 材料导报, 2013, 27(13):54-57.
OU Y P, FAN H Y. Preparation and characterization of a novel adsorbent for coal fly ash[J]. Journal of Hazardous Materials, 2013, 27(13):54-57.
[7] Blissett R S, Rowson N A. A review of the multi-component utilisation of coal fly ash[J]. Fuel, 2012, 97:1-23. doi: 10.1016/j.fuel.2012.03.024
[8] Deng Xin, Qi Liqiang, Zhang Yajuan. Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash[J]. Water, Air and Soil Pollution, 2018, 229:18-23. doi: 10.1007/s11270-017-3679-8
[9] Li Beigang, Yin Haiyang. Preparation, characterization and adsorption application of cerium(III)/fly ash composite materials[J]. Journal of Agro-Environment Science, 2018, 37(9):2005-2013.
[10] Xiyili H, Çetintaş S, Bingöl D. Removal of some heavy metals onto mechanically activated fly ash: Modeling approach for optimization, isotherms, kinetics and thermodynamics[J]. Process Safety and Environmental Protection, 2017, 109:288-300. doi: 10.1016/j.psep.2017.04.012
[11] 许效天, 霍林, 左叶颖, 等. 铝改性粉煤灰漂珠吸附水溶液中砷的性能研究[J]. 中国环境科学, 2011, 31(8):1300-1305.
XU X T, HUO L, ZUO Y Y, et al. Removal of arsenic from aqueous solution by aluminum modified fly ash bleaching beads[J]. China Environmental Science, 2011, 31(8):1300-1305.
[12] 柯昌君, 江盼, 吴维舟. 粉煤灰蒸压活性的红外光谱研究[J]. 武汉理工大学学报, 2009, 31(7):35-39. doi: 10.3963/j.issn.1671-4431.2009.07.010
KE C J, JIANG P, WEI W Z, et al. A study on the autoclaved activity of fly ash by infrared spectroscopy[J]. Journal of Wuhan University of Technology, 2009, 31(7):35-39. doi: 10.3963/j.issn.1671-4431.2009.07.010
[13] 曾丽, 白露, 向莹, 等. 碱性粉煤灰对废水中铬离子吸附性能的研究[J]. 成都大学学报(自然科学版), 2019, 38(2):210-213.
ZENG L, BAI L, XIANG Y, et al. Removal of chromium ions from wastewater by coal fly ash[J]. Journal of Chengdu University (Natural Science Edition), 2019, 38(2):210-213.
[14] 章晓彤, 李芳芹, 任建兴, 等. 钙基吸附剂的改性[J]. 上海电力学院学报, 2018, 34(4):391-394.
ZHANG X T, LI F Q, REN J X, et al. Adsorbents for the removal of calcium from aqueous solution[J]. Journal of Shanghai University of Electric Power, 2018, 34(4):391-394.
[15] 徐洁, 陈海燕, 王旋. 碱改性粉煤灰处理含铬废水[J]. 矿产综合利用, 2016(6):68-71. doi: 10.3969/j.issn.1000-6532.2016.06.016
XU J, CHEN H Y, WANG X, et al. Treatment of chromium containing wastewater by alkali modified fly ash[J]. Multipurpose Utilization of Mineral Resources, 2016(6):68-71. doi: 10.3969/j.issn.1000-6532.2016.06.016
[16] 贺龙强, 付克明, 胡鹏. 改性粉煤灰处理废水中铬(VI)的研究[J]. 煤炭技术, 2018, 37(7):324-326.
HE L Q, FU K M, HU P, et al. Removal of chromium (VI) from wastewater by modified fly ash[J]. Coal Technology, 2018, 37(7):324-326.
-




 下载:
下载: