Natural Evaporation Experiment of Sand Gravel Type Pore Brine in Qaidam Basin in Summer
-
摘要:
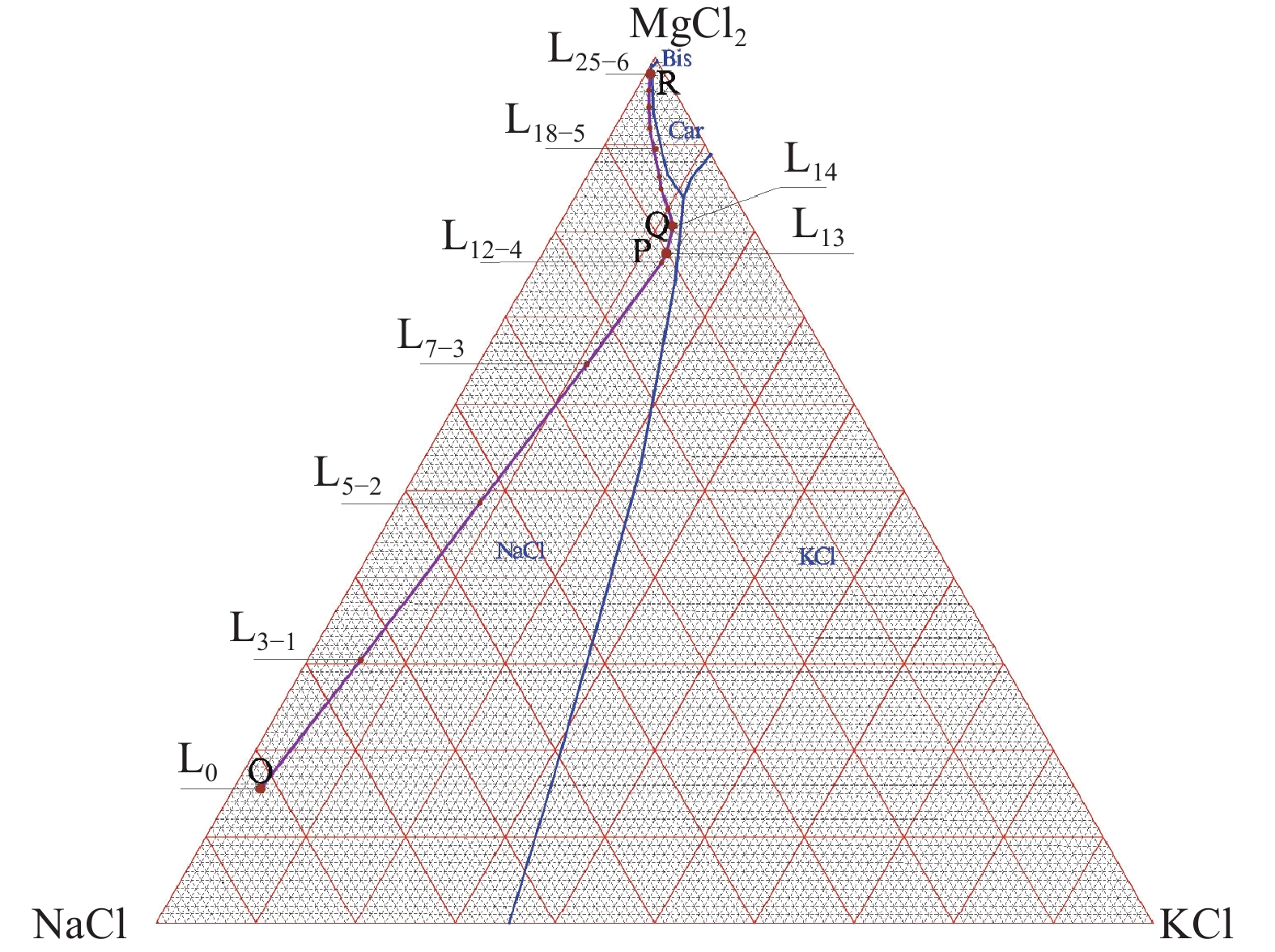
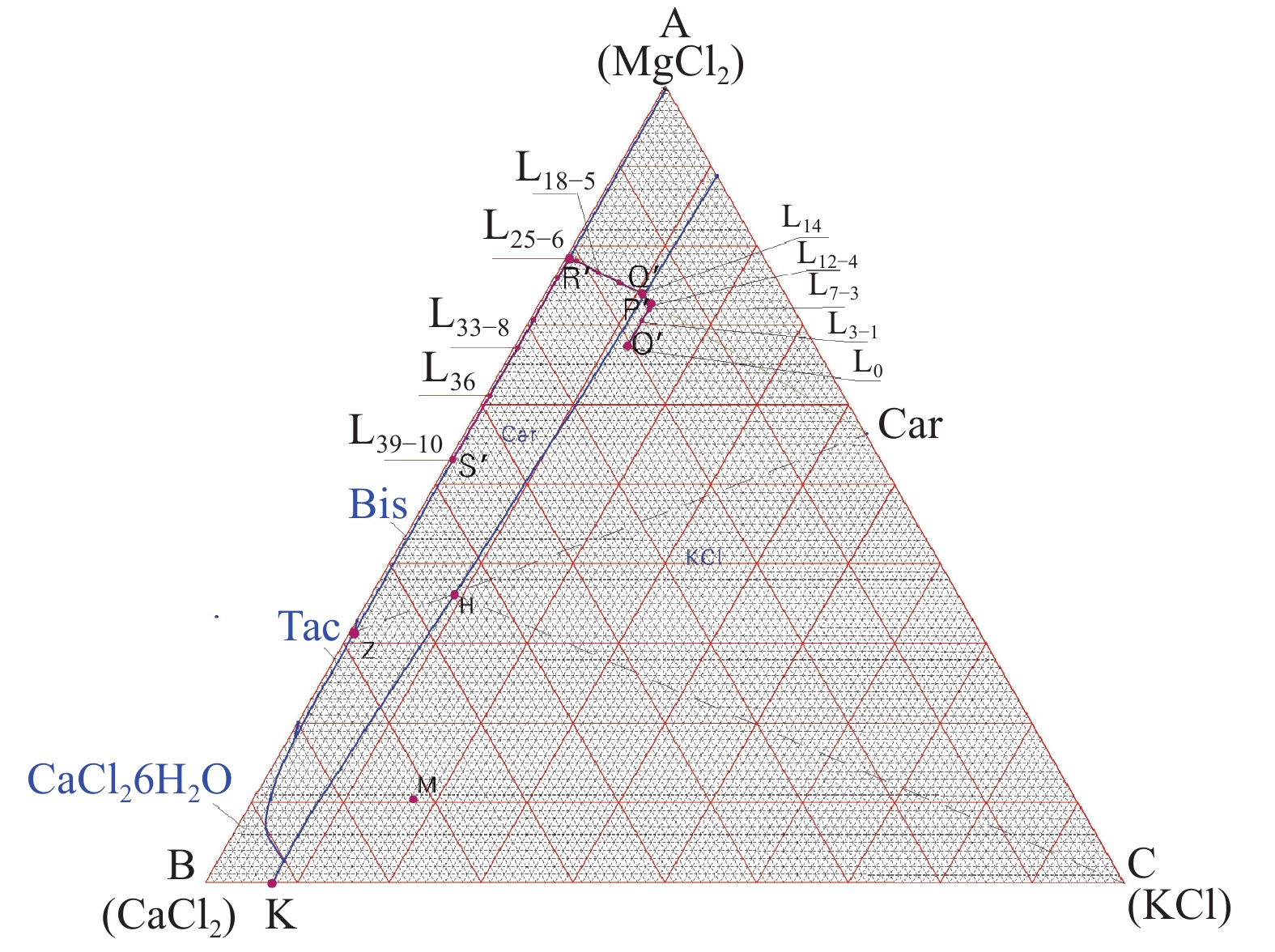
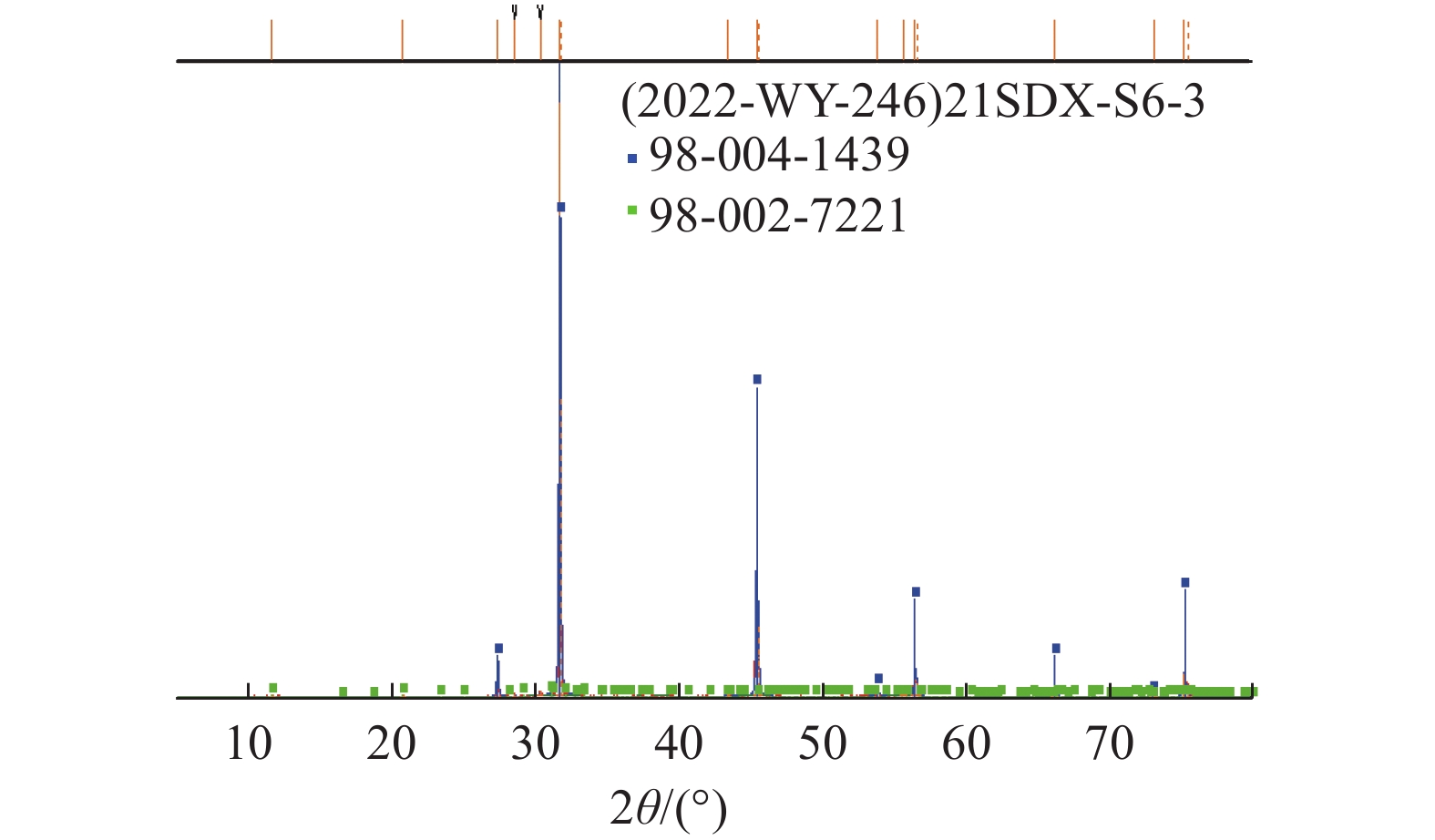
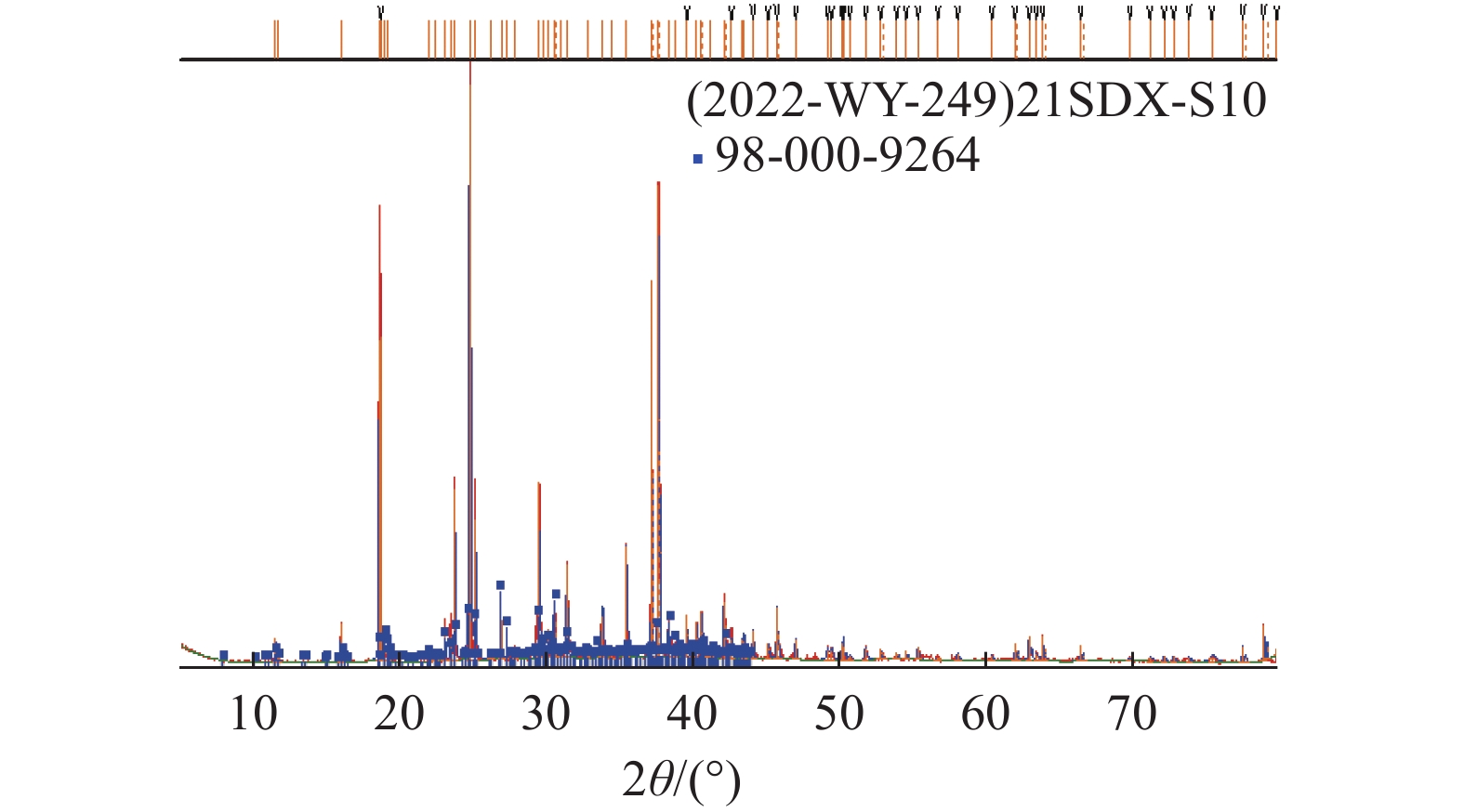
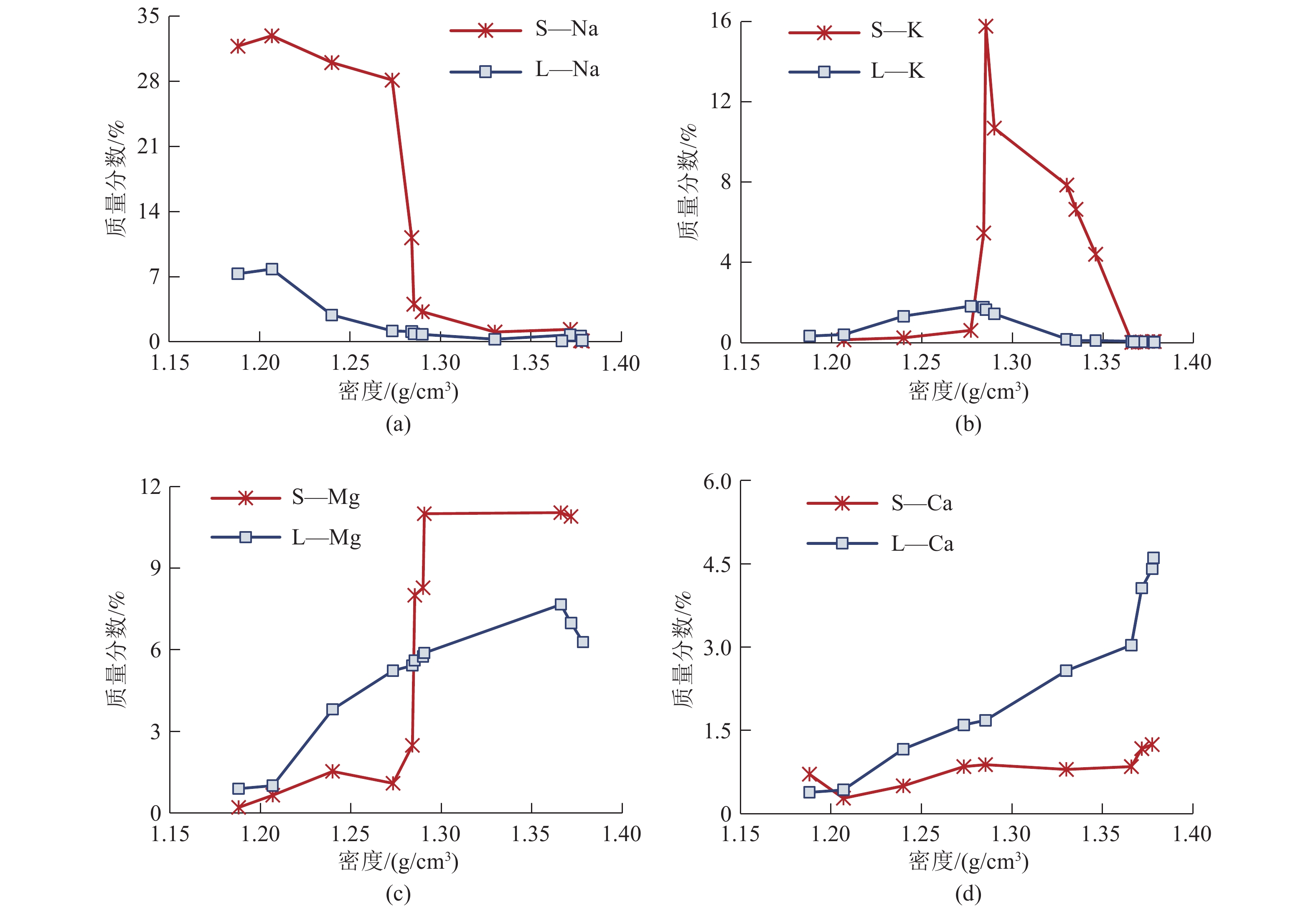
这是一篇矿业工程领域的论文。柴达木盆地深层砂砾型孔隙卤水属高钙、高钠、低钾的不饱和氯化物型卤水,尚未开发。选取大浪滩矿区地下1 000~2 000 m处砂砾型孔隙卤水480 kg,在夏季自然状态下蒸发结晶,分析各离子在固、液相中的分布规律及盐类结晶规律、结晶形态,研究钾的主要析出特点。结果表明:在室外自然状态下蒸发时,析盐过程主要分为石盐→光卤石→水氯镁石→溢晶石四个阶段,其中石盐阶段析盐规律符合Na+,K+,Mg2+//Cl-—H2O 体系25 ℃ 相图,光卤石、水氯镁石阶段符合K+,Ca2+,Mg2+//Cl- —H2O体系25 ℃相图;钾有单独的析出阶段,该阶段钾主要以光卤石形态析出,钾的回收率达85.76%,钾混盐中K+含量9.27%,是生产钾肥的优质原料;钙在水氯镁石阶段之后析出。实验结果可为该类型卤水的开发利用提供参考。
Abstract:This is an article in the field of mining engineering. The deep sand gravel type pore brine in Qaidam Basin belongs to the unsaturated chloride type brine with high calcium, high sodium and low potassium. 480 kg of gravel-type pore brine at 1000~2000 meters underground in Dalangtan mining area was selected for the test, and evaporated and crysta-llized in the natural state in summer. The distribution law of ions in the solid and liquid phases, the salt crystallization law and crystal morphology were analyzed, and the main precipitation characteristics of potassium were studied. The results show when evaporating in the outdoor natural state, the salt precipitation process is mainly divided into four stages: halite→carnallite→bischofite→tachyhydrite. The salt evolution law in the halite stage conforms to the phase diagram of Na+, K+, Mg2+//Cl-—H2O at 25 ℃, and that in carnallite and bischofite stages conforms to the phase diagram of K+, Ca2+, Mg2+//Cl-—H2O system at 25 ℃. There is a separate precipitation stage of potassium, at which potassium is mainly precipitated in the form of carnallite, and the recovery rate of potassium is 85.76%. The K+content in the potassium mixed salt can reach 9.27%, which is a high quality raw material for the production of potassium fertilizer. Calcium precipitates after the brucite stage. The experimental results can provide references for the development and utilization of this type of brine.
-

-
表 1 原始卤水化学组成/(mg/L)
Table 1. Chemical composition of original brines
K+* Na+* Ca2+* Mg2+* Cl+* SO42-* B2O3 Li+ Sr2+ Rb+ Cs+ Br- I- 3.95 87.06 4.57 10.65 177.7 1.90 103.6 5.06 64.34 0.44 0.037 45.57 2.72 *单位为g/L。 表 2 卤水化学组成
Table 2. Chemical composition of brine
编号 离子浓度/(g/L) pH值 密度 卤温 矿化度 相图指数(g/100g S) 相图指数(g/100g S) K+ Na+ Ca2+ Mg2+ Cl- /(g/cm3) /℃ /(g/L) NaCl KCl MgCl2 KCl CaCl2 MgCl2 L0 3.95 87.06 4.57 10.65 177.7 6.82 1.188 18.6 286.0 81.80 2.78 15.42 12.17 20.44 67.39 L1 4.91 94.4 5.13 12.17 197.6 6.39 1.207 28.1 315.9 80.80 3.15 16.05 13.14 19.94 66.92 L3-1 8.25 74.72 7.88 22.56 203.8 6.28 1.213 12.0 319.2 64.60 5.35 30.06 12.49 17.34 70.18 L5-2 12.90 53.44 11.79 36.00 221.7 5.91 1.229 12.0 344.4 45.07 8.16 46.78 12.41 16.47 71.12 L7-3 18.55 31.13 16.44 52.30 248.6 5.56 1.245 39.5 379.7 24.78 11.08 64.15 12.38 15.93 71.69 L11 22.28 15.10 19.10 66.17 270.6 5.05 1.273 23.0 394.5 11.29 12.49 76.22 11.98 14.92 73.10 L12-4 23.37 15.20 20.44 66.93 272.3 5.05 1.277 21.3 398.9 11.19 12.90 75.91 12.27 15.57 72.16 L13 22.88 14.23 20.84 69.55 276.9 5.00 1.284 19.0 405.6 10.27 12.39 77.35 11.67 15.44 72.89 L14 21.07 11.03 21.61 72.10 284.3 4.88 1.285 25.0 411.3 8.00 11.46 80.55 10.50 15.65 73.85 L15 18.63 10.31 23.02 74.31 287.0 4.82 1.290 16.0 414.6 7.43 10.07 82.50 9.10 16.33 74.57 L16 15.12 9.68 24.8 75.89 292.7 4.78 1.291 16.0 419.5 7.02 8.22 84.76 7.30 17.40 75.30 L18-5 9.70 7.11 27.80 79.38 292.9 4.62 1.293 20.9 423.5 5.20 5.32 89.48 4.55 18.94 76.51 L19 7.08 6.78 29.38 83.55 306.8 4.47 1.306 15.0 435.0 4.81 3.77 91.42 3.20 19.27 77.53 L22 2.46 3.81 34.23 91.48 336.5 3.97 1.330 18.0 470.1 2.60 1.26 96.14 1.02 20.70 78.27 L24 1.51 2.19 37.12 98.61 356.1 3.52 1.346 21.0 497.3 1.41 0.73 97.86 0.59 20.89 78.52 L25-6 1.35 2.43 37.77 98.47 360.4 3.59 1.349 21.0 504.8 1.56 0.65 97.78 0.52 21.22 78.26 L27-7 1.20 2.22 37.20 100.2 375.4 3.49 1.354 22.0 518.4 1.41 0.58 98.01 0.47 20.70 78.83 L30 1.01 2.23 45.31 102.5 384.9 3.22 1.368 21.0 538.3 1.39 0.47 98.14 0.36 23.73 75.91 L33-8 0.75 8.92 60.72 89.89 393.5 2.96 1.377 30.0 557.0 6.03 0.38 93.59 0.27 32.23 67.50 L35-9 0.52 2.48 63.48 86.60 380.5 2.63 1.378 17.0 538.4 1.82 0.29 97.89 0.19 34.07 65.74 L36 0.68 1.44 69.58 77.87 355.1 3.20 1.367 -1.0 508.6 1.18 0.42 98.40 0.26 38.61 61.13 L39-10 0.96 1.19 89.46 75.16 383.4 2.49 1.399 18.0 552.1 2.48 0.60 96.92 0.34 45.54 54.13 注:编号中带“-n"样为第n次固液分离时的卤水样。 表 3 析出矿物组成
Table 3. Precipitated mineral composition
编号 卤水密度 盐含量/% /(g/cm3) NaCl CaCl2 KCl MgCl2 CaSO4•2H2O KCl•MgCl2•6H2O MgCl2•6H2O CaCl2•6H2O 合计 S1 1.207 81.59 0.37 0.31 0.82 2.47 - - - 85.56 S3-1 1.213 92.83 0.68 0.17 0.67 1.61 - - - 95.95 S5-2 1.229 95.46 0.28 0.44 1.25 0.77 - - - 98.20 S7-3 1.245 89.68 0.41 0.55 2.43 0.65 - - - 93.72 S11 1.273 83.69 0.69 0.61 3.21 0.22 - - - 88.41 S12-4 1.277 87.55 0.75 1.07 5.01 0.95 - - - 95.33 S13 1.284 70.68 1.27 10.43 4.27 0.18 - - - 86.83 S14 1.285 28.74 2.26 30.07 9.75 0.15 - - - 70.97 S15 1.290 10.18 0.83 - 5.35 0.041 75.97 - - 92.37 S18-5 1.293 19.64 0.97 - 2.40 0.26 70.85 - - 94.12 S22 1.306 10.58 2.40 - 10.72 0.054 55.93 - - 79.69 S23 1.330 11.31 2.90 - 12.68 0.059 47.26 - - 74.20 S24 1.346 13.02 4.23 - 16.50 0.054 31.41 - - 65.21 S25-6 1.349 13.15 2.30 - 10.21 0.22 55.36 - - 81.24 S27-7 1.354 22.93 3.16 - 12.22 0.41 38.53 - - 77.25 S32 1.368 3.33 2.35 - - 0.005 0.30 92.11 - 98.10 S33-8 1.377 4.04 2.14 - - 0.033 0.65 90.89 - 97.74 S35-9 1.378 0.68 7.43 - - 0.020 0.61 83.83 - 92.57 S36 1.367 1.13 - - - 0.004 0.29 5.32 93.21 99.95 S39-10 1.399 1.74 4.56 - - 0.018 0.25 88.15 - 94.72 注:Sx为和前一样品取样间隔内析出盐;Sx-n为第n次固液分离出的混合盐。 表 4 物料汇总
Table 4. Material summary
料别 样号 产品名称 重量 产率 组分含量/% 分布率/% /kg /% K+ Na+ Ca2+ Mg2+ SO42- K+ Na+ Ca2+ Mg2+ SO42- 入 L0 原卤 480.00 100 0.33 7.33 0.38 0.90 0.16 100 100 100 100 100 出(入) L12-4 卤水 78.88 16.43 1.83 1.19 1.60 5.24 0.035 90.35 2.67 68.39 96.04 3.60 出 石盐 91.83 19.13 0.16 36.40 0.51 0.30 0.73 9.21 95.00 25.32 6.41 87.00 失水与损失 309.29 64.44 出(入) L25-6 卤水 41.43 8.63 0.10 0.18 2.80 7.30 0.008 2.59 0.21 62.86 70.28 0.43 出 光卤石 14.78 3.08 9.27 5.94 0.56 7.02 0.15 85.76 2.49 4.49 24.09 2.89 失水与损失 22.67 4.72 合计 97.56 97.71 92.67 100.78 90.32 -
[1] 李洪普, 郭廷峰, 高松, 等. 柴达木西部新近纪以来固液相钾盐资源调查评价报告[R]. 格尔木: 青海省柴达木综合地质矿产勘查院, 2013.LI H P, GUO T F, GAO S, et al. Investigation and evaluation report on solid and liquid potash resources in western qaidam since neogene [R]. Golmud: Qinghai Qaidam Comprehensive Geological and Mineral Exploration Institute, 2013.
LI H P, GUO T F, GAO S, et al. Investigation and evaluation report on solid and liquid potash resources in western qaidam since neogene [R]. Golmud: Qinghai Qaidam Comprehensive Geological and Mineral Exploration Institute, 2013.
[2] 李洪普, 刘国泰, 马宏涛, 等. 青海省茫崖行委黑北凹地液体钾矿详查报告[R]. 格尔木: 青海省柴达木综合地质矿产勘院, 2013.LI H P, LIU G T, MA H T, et al. Detailed investigation report on the liquid potassium mine in Heibei depression of Mangyaxing Commission, Qinghai Province [R]. Golmud: Qinghai Qaidam Comprehensive Geological and Mineral Exploration Institute, 2013.
LI H P, LIU G T, MA H T, et al. Detailed investigation report on the liquid potassium mine in Heibei depression of Mangyaxing Commission, Qinghai Province [R]. Golmud: Qinghai Qaidam Comprehensive Geological and Mineral Exploration Institute, 2013.
[3] 李洪普, 侯献华, 潘彤, 等. 柴达木盆地深层含钾卤水成矿与利用研究[M]. 武汉: 中国地质大学出版社, 2021.10: 6-7.LI H P, HOU X H, PAN T, et al. Mineralization and utilization of deep potassium-bearing brine in the Qaidam Basin [M]. Wuhan: ChinaUniversity of Geosciences , 2021.10: 6-7.
LI H P, HOU X H, PAN T, et al. Mineralization and utilization of deep potassium-bearing brine in the Qaidam Basin [M]. Wuhan: ChinaUniversity of Geosciences , 2021.10: 6-7.
[4] 李洪普, 郑绵平, 侯献华, 等. 柴达木黑北凹地早更新世新型砂砾层卤水水化学特征与成因[J]. 地球科学, 2014, 39(10):1333-1342.LI H P, ZHENG M P, HOU X H, et al. Chemical characteristics and genesis of the brine in the early Pleistocene new gravel layer in the Heibei depression of Qaidam[J]. Earth Science, 2014, 39(10):1333-1342.
LI H P, ZHENG M P, HOU X H, et al. Chemical characteristics and genesis of the brine in the early Pleistocene new gravel layer in the Heibei depression of Qaidam[J]. Earth Science, 2014, 39(10):1333-1342.
[5] 李建森, 李廷伟, 彭喜明, 等. 柴达木盆地西部第三系油田水水文地球化学特征[J]. 石油与天然气地质, 2014, 35(1):50-55.LI J S, LI T W, PENG X M, et al. Hydrogeochemical characteristics of tertiary oilfield water in western Qaidam Basin[J]. Petroleum and Natural Gas Geology, 2014, 35(1):50-55. doi: 10.11743/ogg20140107
LI J S, LI T W, PENG X M, et al. Hydrogeochemical characteristics of tertiary oilfield water in western Qaidam Basin[J]. Petroleum and Natural Gas Geology, 2014, 35(1):50-55. doi: 10.11743/ogg20140107
[6] 靳芳, 李洪普, 常东海. 柴达木盆地南翼山背斜构造区卤水自然蒸发实验[J]. 无机盐工业, 2021, 53(11):86-91.JIN F, LI H P, CHANG D H. Natural evaporation experiment of brine in the south wing mountain anticlinal structural area of Qaidam Basin[J]. Inorganic Salt Industry, 2021, 53(11):86-91.
JIN F, LI H P, CHANG D H. Natural evaporation experiment of brine in the south wing mountain anticlinal structural area of Qaidam Basin[J]. Inorganic Salt Industry, 2021, 53(11):86-91.
[7] 刘颖, 王云生, 乜贞,等. 柴西深层地下卤水资源及其综合利用研究进展[J]. 无机盐工业, 2018, 50(1): 12-18.LIU Y, WANG Y S, NIE Z, et al. Research progress in deep underground brine resources and comprehensive utilization in western Qaidam [J]. Inorganic Salt Industry, 2018, 50 (1): 12-18.
LIU Y, WANG Y S, NIE Z, et al. Research progress in deep underground brine resources and comprehensive utilization in western Qaidam [J]. Inorganic Salt Industry, 2018, 50 (1): 12-18.
[8] 彭玲玲, 魏学斌, 赵为永, 等. 黑北凹地富钾地下卤水自然蒸发实验研究[J]. 无机盐工业, 2019, 51(6):11-16.PENG L L, WEI X B, ZAO W Y, et al. Experimental study on natural evaporation of potassium-rich underground brine in Heibei depression[J]. Inorganic Salt Industry, 2019, 51(6):11-16.
PENG L L, WEI X B, ZAO W Y, et al. Experimental study on natural evaporation of potassium-rich underground brine in Heibei depression[J]. Inorganic Salt Industry, 2019, 51(6):11-16.
[9] 牛自得, 陈芳琴, 李宝存, 等. 水盐体系相图及应用[M]. 天津: 天津大学出版社, 2002.NIU Z D, CHEN F Q, LI B C, et al. Phase diagram of water-salt system and its application [M]. Tianjin: Tianjin University Press, 2002.
NIU Z D, CHEN F Q, LI B C, et al. Phase diagram of water-salt system and its application [M]. Tianjin: Tianjin University Press, 2002.
[10] 陈敬清, 刘子琴, 房春晖, 等. 盐湖卤水的蒸发结晶过程[J]. 盐湖研究, 1994, 2(1):43-51.CHEN J Q, LIU Z Q, FANG C H, et al. Evaporative crystallization process of salt lake brine[J]. Salt Lake Research, 1994, 2(1):43-51.
CHEN J Q, LIU Z Q, FANG C H, et al. Evaporative crystallization process of salt lake brine[J]. Salt Lake Research, 1994, 2(1):43-51.
[11] 杨国彬, 杨建元, 李陇岗, 等. 智利Maricunga盐湖模拟卤水25 ℃等温蒸发实验研究[J]. 盐业与化工, 2012, 41(6):4-8.YANG G B, YANG J Y, LI L G, et al. Experimental study on 25 ℃ isothermal evaporation of simulated brine from Maricunga salt lake in Chile[J]. Salt and Chemical Industry, 2012, 41(6):4-8.
YANG G B, YANG J Y, LI L G, et al. Experimental study on 25 ℃ isothermal evaporation of simulated brine from Maricunga salt lake in Chile[J]. Salt and Chemical Industry, 2012, 41(6):4-8.
-



 下载:
下载: