Research Progress on the Methods Enhancing Biooxidation Pretreatment for Arsenic-Bearing Gold Ores
-
摘要:
微生物预氧化技术具有成本低、设备简单、环境友好等优点,在难处理金矿资源的开发利用中得到了巨大发展和广泛认可。然而,由于原料来源复杂、浸矿菌种耐砷性差、浸矿过程容易生成钝化产物等问题,微生物预氧化在实际生产中依然受到一定程度的制约。目前如何强化含砷金矿微生物浸出已成为该领域的研究热点和难点。综述了目前国内外含砷金矿微生物预氧化强化方法的研究现状,其中详细阐述了应用氧化剂、金属离子、原电池效应、表面活性剂、腐殖酸和磁化水等强化方法的研究进展及作用机理。在此基础上,展望了该领域未来研究的主要发展方向,为含砷金矿微生物预氧化工艺的进一步开发及应用提供参考。
Abstract:Due to the advantages of low cost, simple equipment, and eco-friendliness, biooxidation pretreatment technology has gained great development and wide recognition in the exploitation and utilization of refractory gold ore in recent years. However, some problems involving the complicated properties of crude ores, the low arsenic resistance of microorganisms, and the surface passivation phenomenon restrict the application of biooxidation pretreatment to some degree, so how to accelerate the bioleaching of arsenic-bearing gold ores remains a hot and difficult issue in the field of biohydrometallurgy. This paper summarized the research status of strengthening methods for the biooxidation pretreatment of arsenic bearing gold ores, and elaborated the progress and mechanisms of using oxidants, metal ions, galvanic interaction, surfactants, humid acid and magnetized water. On the basis, the major development directions in the future were prospected, which would provide referential guidance for the further application of biooxidation pretreatment of arsenic-containing gold ores.
-
Key words:
- arsenic-bearing gold ore /
- biooxidation /
- enhancing methods
-

-
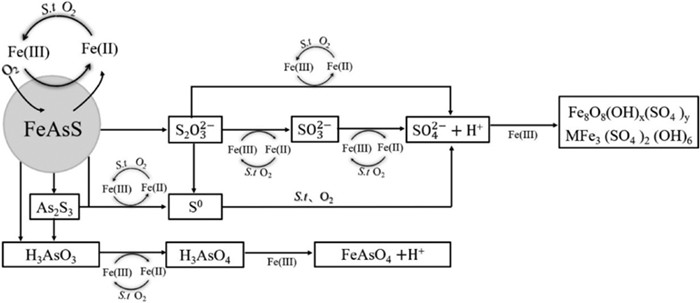
图 1 Fe(Ⅲ)存在条件下S. thermosulfidooxidans菌浸出砷黄铁矿的机理[17](当加入Fe(Ⅲ)时,M为NH4+,而不加入Fe(Ⅲ)时,M为K+)
Figure 1.
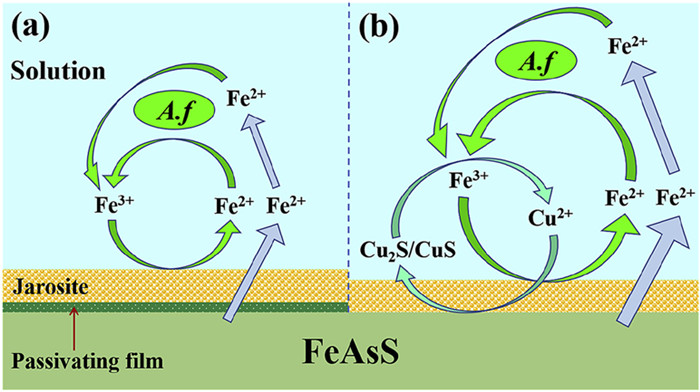
图 2 砷黄铁矿细菌浸出机理示意图[30] (a)不含Cu2+;(b)含Cu2+
Figure 2.
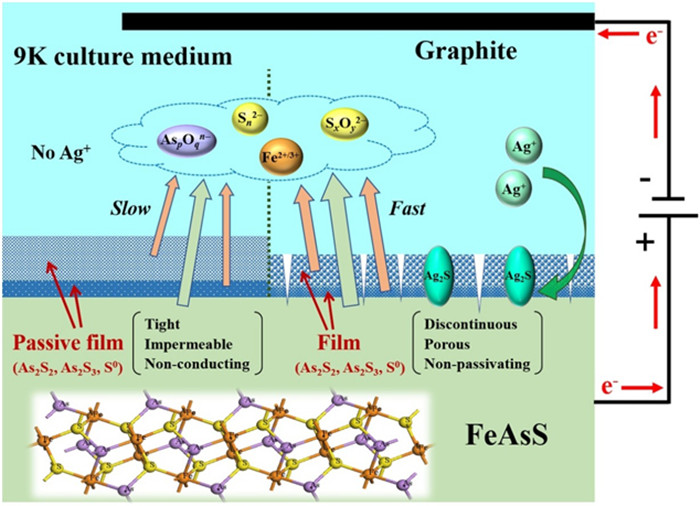
图 3 Ag+对砷黄铁矿电化学氧化溶解的催化机理[33]
Figure 3.
图 4 黄铁矿对砷黄铁矿生物氧化的强化作用机制[49]
Figure 4.
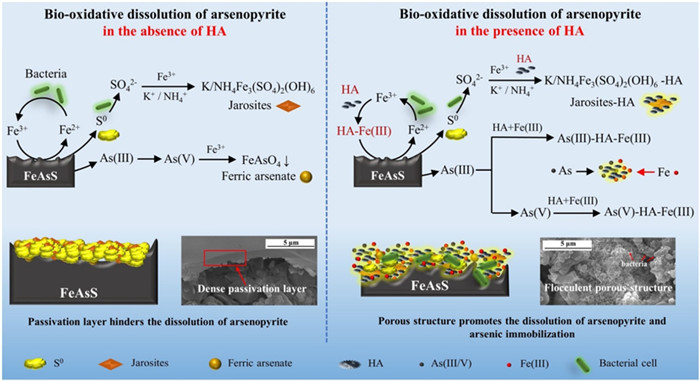
图 5 腐殖酸强化砷黄铁矿微生物浸出机理[60]
Figure 5.
-
[1] MUDD G M. Global trends in gold mining: towards quantifying environmental and resource sustainability[J]. Resources Policy, 2007, 32(1/2): 42-56. https://www.sciencedirect.com/science/article/pii/S0301420707000359
[2] 刘汉钊. 难处理金矿石难浸的原因及预处理方法[J]. 黄金, 1997(9): 44-48. https://www.cnki.com.cn/Article/CJFDTOTAL-HJZZ709.009.htm
[3] MADDOX L M, BANCROFT G M, SCAINI M J, et al. Invisible gold: Comparison of Au deposition on pyrite and arsenopyrite[J]. American Mineralogist, 1998, 83: 1240-1245. doi: 10.2138/am-1998-11-1212
[4] CABRI L J, CHRYSSOULIS S L, De VILLIERS J P R, et al. The nature of "invisible" gold in arsenopyrite[J]. The Canadian Mineralogist, 1989, 27: 353-362. https://www.researchgate.net/publication/258209241_The_nature_of_invisible_gold_in_arsenopyrite
[5] CORKHILL C L, WINCOTT P L, LLOYD J R, et al. The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans[J]. Geochimica et Cosmochimica Acta, 2008, 72(23): 5616-5633. doi: 10.1016/j.gca.2008.09.008
[6] HENAO D M O, GODOY M A M. Jarosite pseudomorph formation from arsenopyrite oxidation using Acidithiobacillus ferrooxidans[J]. Hydrometallurgy, 2010, 104(2): 162-168. doi: 10.1016/j.hydromet.2010.05.012
[7] ZHU T, LU X, LIU H, et al. Quantitative X-ray photoelectron spectroscopy-based depth profiling of bioleached arsenopyrite surface by Acidithiobacillus ferrooxidans[J]. Geochimica et Cosmochimica Acta, 2014, 127: 120-139. doi: 10.1016/j.gca.2013.11.025
[8] MURAVYOV M I, BULAEV A G. Two-step oxidation of a refractory gold-bearing sulfidic concentrate and the effect of organic nutrients on its biooxidation[J]. Minerals Engineering, 2013, 45: 108-114. doi: 10.1016/j.mineng.2013.02.007
[9] MÁRQUEZ M A, OSPINA J D, MORALES A L. New insights about the bacterial oxidation of arsenopyrite: A mineralogical scope[J]. Minerals Engineering, 2012, 39: 248-254. doi: 10.1016/j.mineng.2012.06.012
[10] FOMCHENKO N V, MURAVYOV M I. Thermodynamic and XRD analysis of arsenopyrite biooxidation and enhancement of oxidation efficiency of gold-bearing concentrates[J]. International Journal of Mineral Processing, 2014, 133: 112-118. doi: 10.1016/j.minpro.2014.10.009
[11] FANTAUZZI M, LICHERI C, ATZEI D, et al. Arsenopyrite and pyrite bioleaching: evidence from XPS, XRD and ICP techniques[J]. Analytical and Bioanalytical Chemistry, 2011, 401(7): 2237-2248. doi: 10.1007/s00216-011-5300-0
[12] EDWARDS K J, HU B, HAMERS R J, et al. A new look at microbial leaching patterns on sulfide minerals[J]. FEMS microbiology ecology, 2001, 34(3): 197-206. doi: 10.1111/j.1574-6941.2001.tb00770.x
[13] MIN X B, CHAI L Y, CHEN W L, et al. Bioleaching of refractory gold ore (Ⅱ)-Mechanism on bioleaching of arsenopyrite by Thiobacillus ferrooxidans[J]. Transactions of Nonferrous Metals Society of China, 2002, 12(1): 142-146.
[14] YUNMEI Y, YONGXUAN Z, WILLIAMS-JONES A E, et al. A kinetic study of the oxidation of arsenopyrite in acidic solutions: implications for the environment[J]. Applied Geochemistry, 2004, 19(3): 435-444. doi: 10.1016/S0883-2927(03)00133-1
[15] MIKHLIN Y L, ROMANCHENKO A S, ASANOV I P. Oxidation of arsenopyrite and deposition of gold on the oxidized surfaces: A scanning probe microscopy, tunneling spectroscopy and XPS study[J]. Geochimica et Cosmochimica Acta, 2006, 70(19): 4874-4888. doi: 10.1016/j.gca.2006.07.021
[16] ZHANG D, XIA J, NIE Z, et al. Mechanism by which ferric iron promotes the bioleaching of arsenopyrite by the moderate thermophile Sulfobacillus thermosulfidooxidans[J]. Process Biochemistry, 2019, 81: 11-21. doi: 10.1016/j.procbio.2019.03.004
[17] DENG Y, ZHANG D, XIA J, et al. Enhancement of arsenopyrite bioleaching by different Fe(Ⅲ) compounds through changing composition and structure of passivation layer[J]. Journal of Materials Research and Technology, 2020, 9(6): 12364-12377. doi: 10.1016/j.jmrt.2020.08.088
[18] SANTINI J M, KAPPLER U, WARD S A, et al. The NT-26 cytochrome c552 and its role in arsenite oxidation[J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2007, 1767(2): 189-196. doi: 10.1016/j.bbabio.2007.01.009
[19] 富瑶, 高鹭, 杨洪英, 等. As(Ⅲ)和As(Ⅴ)胁迫下浸矿细菌胞外多糖的变化特征[J]. 金属矿山, 2016(5): 81-84. doi: 10.3969/j.issn.1001-1250.2016.05.018
[20] 向兰, 柯家骏, 裘荣庆. As3+和As5+对细菌生长及含金银的毒砂矿石浸出的影响[J]. 矿产综合利用, 1991(4): 1-5. https://www.cnki.com.cn/Article/CJFDTOTAL-KCZL199104000.htm
[21] 杨松荣, 邱冠周, 胡岳华. As3+及As5+对生物氧化过程的影响及其转化过程的探讨[J]. 国外金属矿选矿, 2003, 40(1): 4-7. https://www.cnki.com.cn/Article/CJFDTOTAL-JSXK200301000.htm
[22] BARRETT J, EWART D K, HUGHES M N, et al. Chemical and biological pathways in the bacterial oxidation of arsenopyrite[J]. FEMS Microbiology Reviews, 1993, 11(1): 57-62. https://www.sciencedirect.com/science/article/pii/0168644593900244
[23] WIERTZ J V, MATEO M, ESCOBAR B. Mechanism of pyrite catalysis of As(Ⅲ) oxidation in bioleaching solutions at 30℃ and 70℃[J]. Hydrometallurgy, 2006, 83(1-4): 35-39. doi: 10.1016/j.hydromet.2006.03.035
[24] 李骞. 含砷金矿生物预氧化提金基础研究[D]. 长沙: 中南大学, 2007.
[25] 李骞, 姜涛, 杨永斌, 等. 一种添加剂在强化细菌氧化含砷金矿上的应用: CN102634661A[P]. 20120815.
[26] CUI R C, YANG H Y, CHEN S, et al. Valence variation of arsenic in bioleaching process of arsenic-bearing gold ore[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1171-1176. doi: 10.1016/S1003-6326(09)60274-0
[27] PATHAK A, MORRISON L, HEALY M G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review[J]. Bioresource Technology, 2017, 229: 211-221. doi: 10.1016/j.biortech.2017.01.001
[28] 苑洪晶. 协同强化含砷金矿生物预氧化的研究[D]. 长沙: 中南大学, 2013.
[29] 李骞, 齐伟, 张雁, 等. Cu2+催化砷黄铁矿生物氧化试验研究[J]. 黄金, 2019, 40(2): 49-54. https://www.cnki.com.cn/Article/CJFDTOTAL-HJZZ201902012.htm
[30] ZHANG Y, LI Q, LIU X, et al. The catalytic effect of copper ion in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans in 9K culture medium[J]. Journal of Cleaner Production, 2020, 256: 120391. doi: 10.1016/j.jclepro.2020.120391
[31] FANG F. Catalytic effect of silver on bioleaching of arsenopyrite[J]. International Journal of Chemical Engineering and Applications, 2014(5): 474-478. http://www.ijcea.org/papers/431-C0004.pdf
[32] 张明, 杨巧文, 张广积, 等. Ag+对含砷金精矿生物浸出的影响[J]. 过程工程学报, 2012, 12(5): 781-784. https://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201205014.htm
[33] ZHANG Y, LI Q, SUN S, et al. Electrochemical behaviour of the oxidative dissolution of arsenopyrite catalysed by Ag+ in 9K culture medium[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 614: 126169. doi: 10.1016/j.colsurfa.2021.126169
[34] CUN-XIONG L I, HONG-SHENG X U, DENG Z G, et al. Pressure leaching of zinc silicate ore in sulfuric acid medium[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 918-923. doi: 10.1016/S1003-6326(09)60236-3
[35] PICH OTERO A, CURUTCHET G, DONATI E, et al. Action of Thiobacillus thiooxidans on sulphur in the presence of a surfactant agent and its application in the indirect dissolution of phosphorus[J]. Process Biochemistry, 1995, 30(8): 747-750. doi: 10.1016/0032-9592(95)00003-E
[36] SIEBERT H M, MARMULLA R, STAHMANN K P. Effect of SDS on planctonic Acidithiobacillus thiooxidans and bioleaching of sand samples[J]. Minerals Engineering, 2011, 24(11): 1128-1131. doi: 10.1016/j.mineng.2011.03.003
[37] 吴爱祥, 艾纯明, 王贻明, 等. 表面活性剂强化铜矿石浸出[J]. 北京科技大学学报, 2013, 35(6): 709-713. https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201306001.htm
[38] LI L, LV Z, YUAN X. Effect of l-glycine on bioleaching of collophanite by Acidithiobacillus ferrooxidans[J]. International Biodeterioration & Biodegradation, 2013, 85: 156-165.
[39] L T D, AND, X M L, et al. Investigations of accelerating parameters for the biooxidation of low-grade refractory gold ores[J]. Minerals Engineering, 2000, 13(14/15): 1543-1553. https://www.sciencedirect.com/science/article/pii/S0892687500001370
[40] FANG, FANG, HONG, et al. Influence of calcium lignosulfonate on bioleaching of arsenic-containing gold concentrate: 2014 International Conference on Environment and Sustainability (ICES 2014)[C].
[41] 李宏煦. 硫化矿细菌浸出过程的电化学机理及工艺研究[D]. 长沙: 中南大学, 2001.
[42] XU J, SHI W, MA P, et al. Corrosion behavior of a pyrite and arsenopyrite galvanic pair in the presence of sulfuric acid, ferric ions and HQ0211 bacterial strain[J]. Minerals, 2019, 9(3): 169. doi: 10.3390/min9030169
[43] TAXIARCHOU M, ADAM K, KONTOPOULOS A. Bacterial oxidation conditions for gold extraction from Olympias refractory arsenical pyrite concentrate[J]. Hydrometallurgy, 1994, 36(2): 169-185. doi: 10.1016/0304-386X(94)90004-3
[44] KOMNITSAS K, XENIDIS A, ADAM K. Oxidation of pyrite and arsenopyrite in sulphidic spoils in Lavrion[J]. Minerals Engineering, 1995(NO. 12): 1443-1454. https://www.sciencedirect.com/science/article/pii/0892687595001093
[45] 杨洪英, 杨立, 赵玉山, 等. 难处理金矿石中硫化物细菌氧化的活性序列[J]. 有色金属工程, 2002, 54(2): 42-44. doi: 10.3969/j.issn.2095-1744.2002.02.011
[46] 崔日成, 杨洪英, 张谷平, 等. 毒砂型高砷金精矿的细菌氧化[J]. 化工学报, 2008(12): 3090-3094. doi: 10.3321/j.issn:0438-1157.2008.12.020
[47] 方芳, 钟宏, 江放明, 等. 黄铁矿对砷黄铁矿生物浸出的影响[J]. 中国有色金属学报, 2013, 23(10): 2970-2976. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201310031.htm
[48] SHA D, GUO H. Catalytic effect of pyrite on the leaching of arsenopyrite in sulfuric acid and acid culture medium[J]. Electrochimica Acta, 2018, 263: 8-16. doi: 10.1016/j.electacta.2018.01.043
[49] SHA D, HE G, WU B, et al. Pyrite-promoted dissolution of arsenopyrite in the presence of Sulfobacillus thermosulfidooxidans[J]. Journal of Materials Research and Technology, 2020, 9(4): 9362-9371. doi: 10.1016/j.jmrt.2020.05.068
[50] PARAMGURU R K, MISHRA K G, KANUNGO S B. Electrochemical phenomena in MnO2-FeS2 leaching in dilute HCl. part 2: studies on polarization measurements[J]. Canadian Metallurgical Quarterly, 1998, 37(5): 395-403. https://www.researchgate.net/publication/222404645_Electro-generative_mechanism_for_simultaneous_leaching_of_pyrite_and_MnO2_in_presence_of_A_ferrooxidans
[51] GANTAYAT B P, RATH P C, PARAMGURU R K, et al. Galvanic interaction between chalcopyrite and manganese dioxide in sulfuric acid medium[J]. Metallurgical and Materials Transactions B, 2000, 31(1): 55-61. doi: 10.1007/s11663-000-0130-z
[52] PARAMGURU R K, NAYAK B B. Galvanic interaction between manganese dioxide and pyrite[J]. Journal of the Electrochemical Society, 1996, 143(12): 3987-3991. doi: 10.1149/1.1837325
[53] MADHUCHHANDA M, DEVI N B, SRINIVASA RAO K, et al. Galvanic interaction between sulfide minerals and pyrolusite[J]. Journal of Solid State Electrochemistry, 2000, 4(4): 189-198. doi: 10.1007/s100080050194
[54] 白云龙, 谢锋, 王伟, 等. 二氧化锰对黄铜矿浸出行为的影响[J]. 有色金属(冶炼部分), 2019(11): 1-6. https://www.cnki.com.cn/Article/CJFDTOTAL-METE201911001.htm
[55] 符剑刚. 采用软锰矿强化辉钼矿的氧化分解及联产钼酸铵与硫酸锰[D]. 长沙: 中南大学, 2009.
[56] 龙怀中. 方铅矿—软锰矿协同浸出直接制备铅、锰氧化物的研究[D]. 长沙: 中南大学, 2013.
[57] ZHAN J, WANG Z, ZHANG C, et al. Separation and extraction of bismuth and manganese from roasted low-grade bismuthinite and pyrolusite: thermodynamic analysis and sulfur fixing[J]. JOM, 2015, 67(5): 1114-1122. doi: 10.1007/s11837-015-1391-y
[58] ZHANG X, FENG Y L, HAO-RAN L I. Enhancement of bio-oxidation of refractory arsenopyritic gold ore by adding pyrolusite in bioleaching system[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2479-2484. doi: 10.1016/S1003-6326(16)64339-X
[59] RASHID, MAMUN, STERBINSKY, et al. Kinetic and mechanistic evaluation of inorganic arsenic species adsorption onto humic acid grafted magnetite nanoparticles[J]. The journal of physical chemistry, C. Nanomaterials and interfaces, 2018: 7b-12438b. https://par.nsf.gov/servlets/purl/10073740
[60] ZHANG D R, CHEN H R, XIA J L, et al. Humic acid promotes arsenopyrite bio-oxidation and arsenic immobilization[J]. Journal of Hazardous Materials, 2019, 384: 121359. https://www.sciencedirect.com/science/article/pii/S0304389419313135
[61] 汪模辉, 邓天龙, 廖梦霞. 含砷金矿的磁场强化生物预氧化[J]. 应用化学, 2000(4): 362-365. doi: 10.3969/j.issn.1000-0518.2000.04.004
[62] YANG B, LIN M, FANG J, et al. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans[J]. Science of The Total Environment, 2020, 698: 134175. doi: 10.1016/j.scitotenv.2019.134175
[63] ZHOU S, GAN M, ZHU J, et al. Catalytic effect of light illumination on bioleaching of chalcopyrite[J]. Bioresour Technol, 2015, 182: 345-352. doi: 10.1016/j.biortech.2015.02.010
[64] 李伟洁. 光及原电池反应对闪锌矿微生物氧化作用的影响[D]. 南京: 南京大学, 2020.
[65] YANG B J, LUO W, LIAO Q, et al. Photogenerated-hole scavenger for enhancing photocatalytic chalcopyrite bioleaching[J]. Transactions of Nonferrous Metals Society of China, 2020, 30(1): 200-211. doi: 10.1016/S1003-6326(19)65192-7
-



 下载:
下载: