Effect of Exposure Levels and Exposure Time on Distribution of Cadmium Species in Indian Mustard (Brassica Juncea)
-
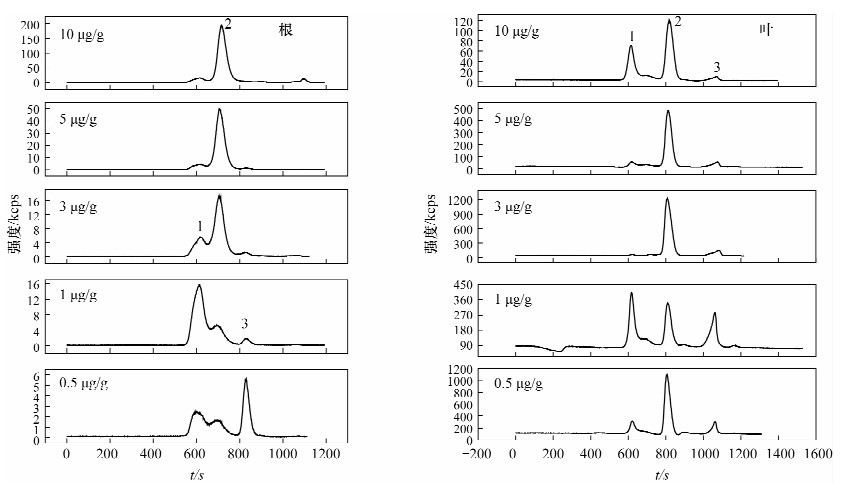
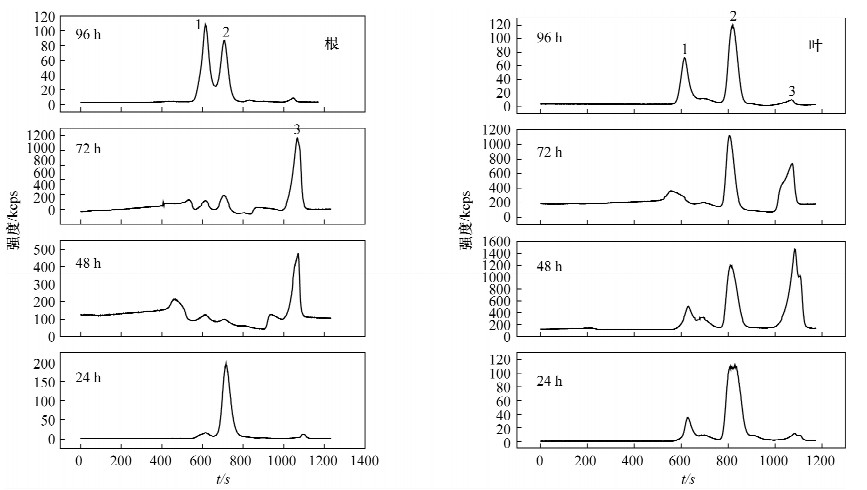
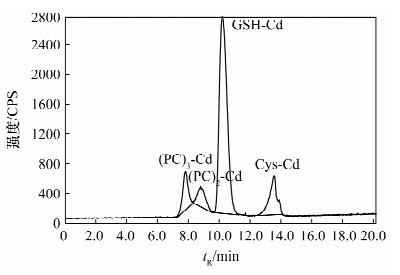
摘要: 螯合作用是植物对细胞内重金属耐受的主要方式之一,植物中螯合肽(PCs)在植物耐重金属毒害中的作用己有许多报道,但作用程度并未得出一致的结论,关于PCs是在镉刺激下直接合成还是以谷胱甘肽为底物合成同样存在争议。本文研究了胁迫浓度和胁迫时间对超积累植物印度芥菜(Brassica juncea)中镉形态分布的影响。印度芥菜幼苗分别用0.5、1.0、3.0、5.0、10.0 mg/L镉标准溶液胁迫24 h、48 h、72 h、96 h后收获,用体积排阻高效液相色谱-电感耦合等离子体质谱技术测定植物体根部和叶部中镉形态的含量。结果表明,在低胁迫浓度下(≤3.0 mg/L),植物叶中PCs-Cd的含量与胁迫浓度成正比;在高胁迫浓度下,PCs-Cd含量反而降低,根中PCs-Cd的含量持续增加,但叶中PCs-Cd总量高于根部,说明PCs在植物体内会由根部向叶部转移,从而提高了镉耐受性。在持续长时间胁迫下,PCs-Cd含量也降低,表明PCs在镉解毒机制中仅有短暂的作用;持续高浓度胁迫下,植物会引发其他机制来抵制Cd的毒性。研究认为PCs在镉解毒机制中的作用需要考虑胁迫时间和胁迫浓度这两个重要参数。Abstract: Chelation is one of the main ways to tolerate the heavy metals in the cells of plants. Phytochelatin (PCs) was reported to have detoxification and compartmentalization of heavy metals, but no accordant conclusion for its contribution. Whether the PCs were directly synthetized under the stimulation of Cd or synthetized by glutathione is still an unsolved issue. In this paper, a study is described examining the relationship between exposure levels, exposure time and Cd tolerance. Root and leaf samples were placed in 0.5, 1.0, 3.0, 5.0 and 10.0 mg/L Cd standard solutions and harvested after 24, 48, 72, 96 hours. The Cd species in the root and leaf samples were measured by using Size-Exclusion High-Performance Liquid Chromatography and Inductively Coupled Plasma-Mass Spectrometry. Results indicated that PCs-Cd contents were positively correlated with Cd exposure levels when the lower Cd exposure levels were less than 3.0 mg/L. Under the higher exposure levels, the contents of PCs-Cd were reduced, the PCs-Cd contents in root samples were continually increasing, but lower than those found in the leaf samples, which indicated that the PCs were transported from root to leaf with a higher tolerance of Cd. Increasing exposure time also reduced PCs-Cd production which indicated PCs may only have a temporary role in metal resistance. Under continuous higher exposure, plants may trigger other mechanisms that tolerate heavy metal toxicity. Our results suggest that concentration and time of exposure are important factors that must be taken into consideration when evaluating the true role of PCs in heavy metal detoxification.
-
Key words:
- Indian mustard /
- cadmium species /
- exposure levels /
- exposure time /
- tolerance
-

-
[1] Clemens S, Palmgren M G, Kramer U. A long way ahead: Understanding and engineering plant metal accumulation [J].Trends in Plant Science,2002,7(7): 309-315. doi: 10.1016/S1360-1385(02)02295-1
[2] Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants [J].Biochimie,2006, 88(11): 1707-1719. doi: 10.1016/j.biochi.2006.07.003
[3] Hall J L. Cellular mechanisms for heavy metal detoxification and tolerance [J].Journal of Experimental Botany, 2002, 53(366): 1-11.
[4] Yang X E, Feng Y, He Z L, Stoffella P J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation [J].Journal of Trace Elements in Medicine and Biology, 2005,18(4): 339-353. doi: 10.1016/j.jtemb.2005.02.007
[5] Belcastro M, Marino T, Russo N, Toscano M. The role of glutathione in cadmium ion detoxification: Coordination modes and binding properties—A density functional study[J].Journal of Inorganic Biochemistry, 2009, 103(1): 50 57. doi: 10.1016/j.jinorgbio.2008.09.002
[6] Küpper H, Lombi E, Zhao F J, McGrath S P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri [J].Planta, 2000, 212(1): 75-84. doi: 10.1007/s004250000366
[7] Salt D E, Prince R C, Pickering I J. Chemical speci-ation of accumulated metals in plants: Evidence from X-ray absorption spectroscopy[J].Microchemical Journal,2002, 71(2-3): 255-259. doi: 10.1016/S0026-265X(02)00017-6
[8] Sarret G, Saumitou-Laprade P, Bert V, Proux O, Hazemann J L, Traverse A, Marcus M A, Manceau A. Forms of Zinc accumulated in the hyperaccumulator Arabidopsis halleri [J].Plant Physiology,2002,130(4): 1815-1826. doi: 10.1104/pp.007799
[9] Sarret G, Willems G, Isaure M P, Marcus M A, Fakra S C, Frérot H, Pairis S, Geoffroy N, Manleau A, Saumitou-Laprade P. Zinc distribution and speciation in Arabidopsis halleri x Arabidopsis lyrata progenies present-ing various zinc accumulation capacities [J].New Phytologist, 2009, 184(3): 581-595. doi: 10.1111/nph.2009.184.issue-3
[10] Callahan D L, Baker A J, Kolev S P, Wedd A G. Metal ion ligands in hyperaccumulating plants [J].Journal of Biological Inorganic Chemistry, 2006,11(1): 2-12. doi: 10.1007/s00775-005-0056-7
[11] Haydon M J, Cobbett C S. Transporters of ligands for essential metal ions in plants [J].New Phytologist, 2007, 174(3): 499-506. doi: 10.1111/nph.2007.174.issue-3
[12] Rauser W E. The role of thiols in plants under metal stress[M]//Brunold C,eds. Sulphur Nutrition and Sulphur Assimilation in Higher Plants. Switzerland: Paul Haupt Bern, 2000: 169-183.
[13] Wei Z G, Wong J W, Zhao H Y, Zhang H J, Li H X, Hu F. Separation and determination of heavy metals associated with low molecular weight chelators in xylem saps of Indian mustard (Brassica juncea)by size exclusion chromatograph and atomic absorption spectrometry [J].Biological Trace Element Research, 2007, 118(2): 146-158. doi: 10.1007/s12011-007-0022-z
[14] di Toppi L S, Gabrielli R. Response to cadmium in higher plants[J].Environmental and Experimental Botany, 1999, 41(2): 105-130. doi: 10.1016/S0098-8472(98)00058-6
[15] Leopold I, Schmide J, Neuman D, Günther D. Phytoche-latins and heavy metal tolerance[J].Phytochemistry, 1999, 50(8): 1323-1328. doi: 10.1016/S0031-9422(98)00347-1
[16] Piechalack A, Tomaszewska B, Baralkiewicz D, Malecka A. Accumulation and detoxification of lead ions in legumes[J].Phytochemistry, 2002, 60(2): 153-162. doi: 10.1016/S0031-9422(02)00067-5
[17] Zhao F J, Wang J R, Barker J H A, Schat H, Bleeker P M, McGrath S P. The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata [J].New Phytologist, 2003, 159(2): 403-410. doi: 10.1046/j.1469-8137.2003.00784.x
[18] Verbruggen N, Hermans C, Schat H. Molecular mechan-isms of metal hyperaccumulation in plants [J].New Phytologist, 2009, 181(4): 759-776. doi: 10.1111/j.1469-8137.2008.02748.x
[19] Ebbs S, Lau I, Ahner B, Kochian L. Phytochelatin synthesis is not responsible for Cd tolerance in the Zn/Cd hyperaccumulator Thlaspi caerulescenes (J. and C. Presl) [J].Planta,2002, 214(4): 635-640. doi: 10.1007/s004250100650
[20] Sun Q, Ye Z H, Wang X R, Wong M H. Increase of glutathione in mine population of Sedum alfredii: A Zn hyperaccumulator and Pb accumulator [J].Phytochemistry, 2005,66(20): 2549-2556.
[21] Sun Q, Ye Z H, Wang X R, Wong M H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyper-accumulator Sedum alfredii [J].Journal of Plant Physiology,2007, 164(11): 1489-1498. doi: 10.1016/j.jplph.2006.10.001
[22] Cobbett C S. A family of phytochelatin synthase genes from plant fungal and animal species[J].Trends in Plant Science, 1999, 4(9): 335-337. doi: 10.1016/S1360-1385(99)01465-X
[23] Grill E, Winnacker E L, Zenk M H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants [J].Science, 1985, 230(4726): 674-676. doi: 10.1126/science.230.4726.674
[24] Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker P M. The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes [J].Journal of Experimental Botany, 2002,53(379): 2381-2392. doi: 10.1093/jxb/erf107
[25] Yang H X, Liu W, Li B, Wei W, Zhang H J, Chen D Y.Speciation analysis of Cadmium in Indian mustard (Brassica Juncea) by size exclusion chromatography- high performance liquid chromatography-inductively coupled plasma mass spectrometry (SEC-HPLC-ICP-MS) [J].Chinese Journal of Analytical Chemistry,2009,37(10): 1511-1514. doi: 10.1016/S1872-2040(08)60137-1
[26] 杨红霞,刘崴,李冰,魏巍,张惠娟,陈登云.体积排阻高效液相色谱-电感耦合等离子体质谱法同时测定印度芥菜(Brassica Juncea)中镉铜锌形态[J].岩矿测试,2010, 29(1): 9-13.
-



 下载:
下载: